Weaning and Discontinuation from Mechanical Ventilation
Learning Objectives
On completion of this chapter, the reader will be able to do the following:
1 List the weaning parameters and acceptable values for ventilator discontinuation.
3 Define the closed loop modes of weaning described in the chapter.
6 Describe criteria used to determine whether a patient is ready for extubation.
7 Recognize postextubation difficulties from a clinical case description.
8 Recommend appropriate treatment for postextubation difficulties.
11 Name the parameter used as the primary index of drive to breathe.
16 Assess data to establish the probable cause of failure to wean.
Key Terms
• Adaptive support ventilation
• Automatic tube compensation
• Mandatory minute ventilation
• Respiratory alternans
• Weaning
Weaning Techniques
Patients require mechanical ventilation when their ability to support ventilatory demands is outweighed by a disease process or when the respiratory drive is inadequate to maintain ventilation because of disease or medications (Fig. 20-1).1,2 Once the need for mechanical ventilation has been resolved, ventilation can be discontinued. This is a simple maneuver for most patients, for whom the ventilator is simply disconnected from the patient and the endotracheal tube (ET) is removed. About 80% of patients requiring temporary mechanical ventilation do not require a gradual withdrawal process and can be disconnected within a few hours or days of initial support.3,4 Examples of this type of temporary ventilation include postoperative ventilatory support for recovery from anesthesia and treatment of uncomplicated drug overdose and exacerbations of asthma. When the patient has undergone ventilation for less than a week, discontinuation is usually a quick process. However, for a some patients, the process can be lengthy and complex.5
The term weaning is frequently used to describe the gradual reduction of ventilatory support from a patient whose condition is improving.1,2,4–7 Some practitioners prefer terms such as discontinuation, gradual withdrawal, or liberation (Key Point 20-1).1 Regardless of the terminology, the process is the same.
Several facts must be taken into consideration if ventilation is to be discontinued successfully. First, some patients require ventilatory support during weaning. Second, oxygen and positive end-expiratory pressure (PEEP) may be required to support oxygenation. Third, some individuals may require maintenance of the artificial airway even after ventilatory support has been discontinued. Fourth, many patients require more than one of the preceding therapies. Although each of the first three components can be treated separately, the overall process of ventilator discontinuation involves all of them (Box 20-1).6,7
Ventilatory support should be discontinued and the artificial airway removed as soon as possible to avoid the risks associated with mechanical ventilation, such as ventilator-induced lung injury, ventilator-associated pneumonia (VAP), airway trauma from the ET, and unnecessary sedation. On the other hand, premature withdrawal of ventilatory support or of the airway can result in ventilatory muscle fatigue, compromised gas exchange, and loss of airway protection.1 Premature discontinuation is also associated with a higher mortality rate.8–10
The decision to wean a patient from the ventilator depends on the patient’s level of recovery from the medical problems that imposed the need for mechanical ventilation and the patient’s overall clinical condition and psychological state. Therefore the patient’s physiological capacity and mental and emotional condition must be evaluated before an attempt is made to remove the patient from ventilatory support.
This chapter reviews ventilator techniques used during weaning from ventilatory support, as well as evidence-based recommendations for determining whether a patient meets the criteria for ventilator discontinuation. A discussion of the process of weaning, clinical conditions that may compromise a patient’s ability to be weaned, and the introduction to long-term care when a patient cannot be weaned are also presented.
Methods of Titrating Ventilator Support During Weaning
Ventilator support can be reduced as patients become increasingly able to resume part of the work of breathing (WOB). Three approaches have been commonly used to reduce ventilatory support and gradually place more of the WOB on the respiratory muscles: synchronized intermittent mandatory ventilation (SIMV), pressure support ventilation (PSV), and T-piece weaning. Until the early 1990s, the three methods were considered equally effective.11 More recent studies have clearly shown that the weaning process was inordinately prolonged with SIMV compared with other weaning techniques.10,12 Despite these findings, a substantial number of physicians continue to use SIMV to wean patients from mechanical ventilatory support.13
In addition to these traditional methods, more sophisticated forms of closed-loop ventilation have been introduced for weaning patients. These include volume-targeted, PSV (e.g., volume support), automode, mandatory minute ventilation (MMV), automatic tube compensation, and artificial intelligence systems.
Synchronized Intermittent Mandatory Ventilation
SIMV began as a method to synchronize a patient’s efforts with the mandatory breaths provided by the ventilator during intermittent mandatory ventilation (IMV). The theory underlying IMV/SIMV is that the patient’s respiratory muscles would work during spontaneous breathing intervals and rest during mandatory breaths.14
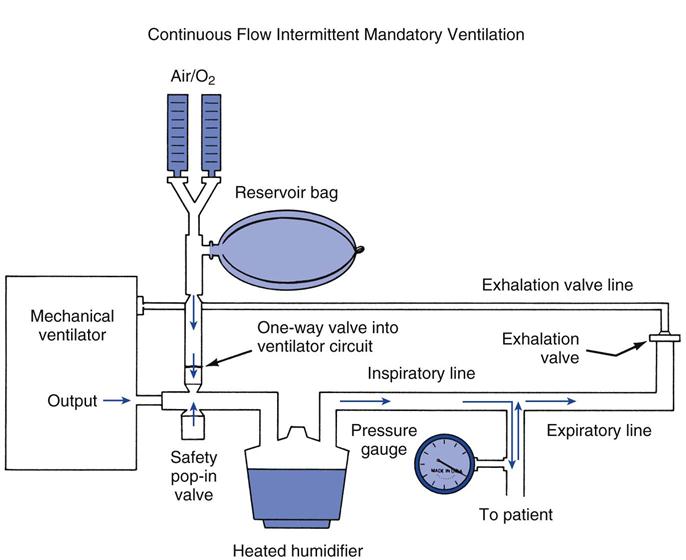
Intermittent mandatory ventilation (IMV) was first introduced in the 1960s as a method to ventilate infants afflicted with idiopathic respiratory distress syndrome (NOTE: The term idiopathic was originally used to describe infant respiratory distress syndrome because the cause was unknown at the time.) The first IMV systems used to wean adult patients from mechanical ventilation were introduced in the 1970s. Figure 20-2 shows a diagram of a volume ventilator with an added continuous-flow IMV circuit used for adult patients. A blended gas source is directed into a reservoir bag (3 L anesthetic bag). The IMV circuit connects this reservoir to the ventilator circuit by means of a one-way valve. The one-way valve prevents a positive-pressure breath, generated by the machine, from entering the reservoir bag. This system allows a continuous flow of gas from the reservoir bag through the humidifier and the main inspiratory line to the patient. During a positive-pressure breath, the high pressure closes the one-way valve, preventing machine air from entering the reservoir bag and allowing the mandatory ventilator breath.
A common weaning practice with SIMV is to reduce the mandatory rate progressively, usually in steps of 1 or 2 breaths/min and at a pace that matches the patient’s improvement. PSV can be added to unload the spontaneous breaths and reduce the patient’s WOB through the ventilator system, circuit, and artificial airway, which in turn can help prevent excessive fatigue (Case Study 20-1). Use of pressure support is especially important when the SIMV rate is low (i.e., <4-6 breaths/min). The level of PSV used during SIMV typically ranges from 5 to 10 cm H2O; the set pressure usually depends on assessment of the spontaneous tidal volume (VT) achieved and the apparent work of breathing. PEEP of 3 to 5 cm H2O is also used to help compensate for changes in functional residual capacity (FRC) associated with the use of an ET and the recumbent position.15,16
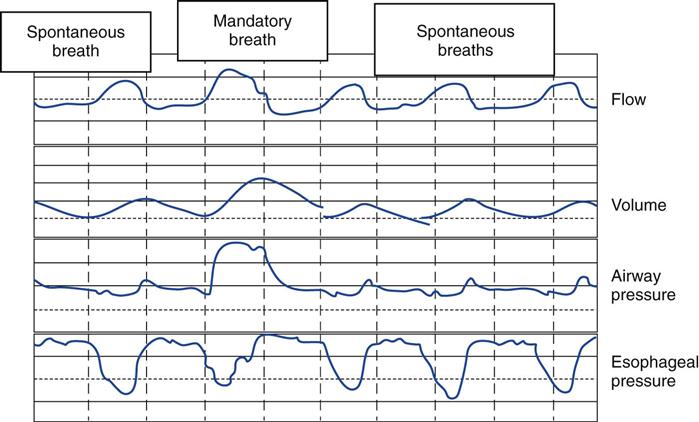
In reality, the respiratory muscles may perform significant work with both mandatory and spontaneous breaths during SIMV (Fig. 20-3). Ventilator asynchrony may occur because the patient’s respiratory center does not anticipate whether the next breath from the ventilator will be mandatory or spontaneous.15 When the mandatory rate is reduced to the point where it provides 50% or less of the required minute ventilation ( ), the WOB for the patient actually may be as much as when support is withdrawn completely.16,17 Consequently, the patient’s spontaneous respiratory rate may increase significantly.10,12 (Additional information on SIMV can be found in Chapter 5.)
), the WOB for the patient actually may be as much as when support is withdrawn completely.16,17 Consequently, the patient’s spontaneous respiratory rate may increase significantly.10,12 (Additional information on SIMV can be found in Chapter 5.)
Pressure-Support Ventilation
With PSV, the patient controls the rate, timing, and depth of each breath; in other words, PSV is patient triggered, pressure limited, and flow cycled. The sophisticated monitoring and alarm systems on current intensive care unit (ICU) ventilators make this non–volume-oriented approach a safe, effective mode of weaning. Theoretically, PSV allows the clinician to adjust the ventilatory workload for each spontaneous breath to enhance endurance conditioning of the respiratory muscles without causing fatigue.17,18
The most practical method of establishing the level of PSV is to base the initial setting on the patient’s measured airway resistance. In general, this is a pressure level of 5 to 15 cm H2O in patients who meet weaning criteria. Another sound approach involves attempting to reestablish a patient’s baseline respiratory rate (15-25 breaths/min) and VT (300 to 600 mL/min). An inappropriate PSV setting can be identified by the presence of respiratory distress, which manifests as tachycardia, hypertension, tachypnea, diaphoresis, paradoxical breathing, respiratory alternans (altering use of the diaphragm to breath and the accessory muscles of respiration), and excessive accessory muscle use.
During weaning with PSV, the clinician gradually reduces the level of support as long as an appropriate spontaneous respiratory rate and VT are maintained and distress is not evident. When pressure support is reduced to about 5 cm H2O, the pressure level is not high enough to contribute significantly to ventilatory support. However, this level of support is usually sufficient to overcome the work imposed by the ventilator system (i.e., the resistance of the ET, trigger sensitivity, demand-flow capabilities, and the type of humidifier used).
T-Piece Weaning
T-piece weaning is the oldest of the available techniques. It originally involved removing the ventilator from the patient according to a predetermined schedule. The weaning process started when the patient was able to breathe spontaneously for brief periods without ventilatory support and the criteria for weaning had been met (these criteria are discussed later in the chapter). The original T-piece trial followed a schedule that progressively increased the length of time the patient was removed from ventilatory support. For example, the first period might have been 5 to 10 minutes, after which the patient was returned to the ventilator for the remainder of the hour. This process was repeated once an hour. The time off the ventilator was increased gradually until the patient was off the ventilator for 30 minutes and on for 30 minutes. The off time then was increased to 1 hour and so on.
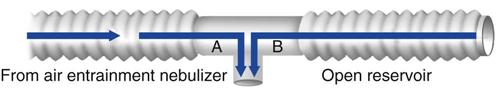
The setup for a T-piece system includes a heated humidifier with a large reservoir. The humidifier is connected to a blended gas source (air/oxygen) that provides a high flow of gas (at least 10 L/min) at the desired fractional inspired oxygen (FIO2). The humidified gas source is connected to a T-piece (Briggs adapter) with large-bore tubing, which is attached to the patient’s ET. Another piece of large-bore tubing is attached to the exhalation side of the T-piece (volume of about 120 mL) to provide a reservoir or, as some clinicians refer to it, an afterburner (Fig. 20-4).19 If the patient inhales and the gas flow through the tubing from the humidifier is inadequate, some of the patient’s inhaled air can be derived from this reservoir and still contain gas at the desired FIO2. Patients are seated or semi-recumbent for the procedure. They also are monitored continuously by a clinician while disconnected from the ventilator, thus requiring a high level of staff attention.
When T-piece weaning is accomplished through the ventilator, the ventilatory mode is set to spontaneous/continuous positive airway pressure (CPAP); that is, the mandatory rate is turned off. The advantage of using the ventilator is the availability of alarms; the disadvantage is that the patient’s efforts to breathe through the ventilator system may result in an increased workload. However, current ICU ventilators typically provide a bias flow of gas through the system and flow-triggering that supports any spontaneous breaths and reduces the patient’s WOB. Basically, this is similar to a small amount of pressure support. Thus the T-piece trial using a ventilator provides a means of continuously monitoring the patient. This approach also provides backup apnea alarms or backup ventilator modes to support patients who become apneic.
Patients less likely to tolerate T-piece weaning include those who have severe underlying heart disease or severe muscle weakness or who are inclined to panic because of psychological problems or preexisting chronic lung conditions.
Comparison of Traditional Weaning Methods
Studies comparing the T-piece, SIMV, and PSV weaning techniques have produced conflicting results.10,12 Each mode offers particular benefits to certain patients. If one procedure does not work well for a patient, another might work. Ventilator discontinuation is best accomplished when expert, caring staff members work with willing, cooperative patients.
Closed-Loop Control Modes for Ventilator Discontinuation
In a closed-loop control ventilatory mode, a set variable is compared with a measured control variable.20 The ventilator uses a feedback signal to adjust the output of the system. Closed-loop modes of ventilation range from simple techniques, such as volume support, to more complex ones, such as adaptive support ventilation. Advanced closed-loop control techniques that have been used for weaning include automatic tube compensation (ATC), volume-targeted PSV (e.g., volume support), mandatory minute ventilation (MMV), adaptive support ventilation (ASV), and an artificial intelligence system for weaning.
Automatic Tube Compensation
The WOB may increase when a spontaneously breathing patient breathes unaided through an ET.20–22 The amount of the increase is directly related to the size of the artificial airway and the  . Reducing the diameter or increasing the length of the tube increases the resistance to flow, as do kinks in the tube. These factors, coupled with high
. Reducing the diameter or increasing the length of the tube increases the resistance to flow, as do kinks in the tube. These factors, coupled with high  , increase the spontaneous WOB for the patient (see Chapter 17).
, increase the spontaneous WOB for the patient (see Chapter 17).
Clinicians often use low levels of pressure support (<10 cm H2O) to compensate for the increased resistance and WOB associated with breathing through an ET.21–23 However, the clinician must always keep in mind that a fixed pressure, as with PSV, cannot accurately compensate for the variable flow through the ET because inspiratory flow demand can vary.24 Therefore a fixed level of pressure support can result in too little support when inspiratory flow is high or too much support when inspiratory flow is low.25 In fact, PSV can result in excessive VT and flow, which is uncomfortable for the patient.26
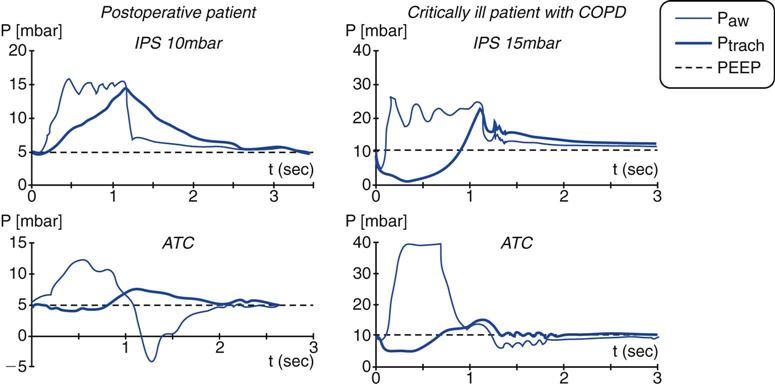
To overcome this problem, some ICU ventilators (e.g., Puritan Bennett 840 [Covidien-Nellcor Puritan-Bennett, Boulder, Colo.], Dräger Evita XL [Dräger Medical, Telford, Pa]) are equipped with a feature called automatic tube compensation (ATC). ATC was designed specifically to reduce the WOB associated with increased ET resistance.15 Theoretically, ATC delivers exactly the amount of pressure required to overcome the resistive load imposed by the ET for the flow measured at the time. In a sense, this is providing variable PSV with variable inspiratory flow compensation.24 ATC targets pressure at the tracheal level, adjusting the delivered pressure to try to maintain tracheal pressure at a constant level (Fig. 20-5).27,28 If the flow-resistive properties of the artificial airway are known, tracheal pressure changes can be determined by measuring inspiratory and expiratory flows.29
Automatic tube compensation functions by using a closed-loop control of the ventilator based on calculated tracheal pressures.27,30 ATC increases the pressure at the upper airway by an amount equal to the continuously calculated pressure drop across the ET during inspiration.29 The pressure change required to maintain the flow for a known resistance can be estimated using the following equation:
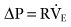
where ΔP is the pressure change, R is resistance, and 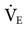 is flow. The equation for calculating tracheal pressure31 is
is flow. The equation for calculating tracheal pressure31 is
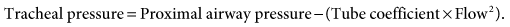
In this equation, the tube coefficient relates to the size of the tube and its imposed resistance. (NOTE: ET resistance is a nonlinear function of flow, especially at higher flows.29,32)
To set ATC, the operator selects the ATC function on the ventilator and enters the type of tube (ET or tracheostomy) and the tube size. Some ventilators allow selection of both inspiratory and expiratory ATC.31,33 Currently, it is unclear whether expiratory ATC might cause premature closure of unstable airways in patients with chronic obstructive pulmonary disease (COPD)28 or whether it might, in fact, eliminate dynamic hyperinflation.34 Further clinical studies are needed to evaluate this issue.
In relation to WOB, ATC may support spontaneous breathing without the overcompensation or undercompensation that occurs with PSV or CPAP.28,31,35 In addition to the benefit of reduced WOB, patients seem to find ATC more comfortable than PSV.26,36 (The respiratory discomfort in PSV seems to be related to lung overinflation.26,27,36) Box 20-2 lists the potential benefits of ATC.34,37,38
Arguments Against the Use of Automatic Tube Compensation
Some question whether ATC is needed to support a spontaneously breathing, intubated patient. Until a patient’s spontaneous 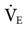 exceeds 10 L/min, the effect of the ET might not be significant (see Fig. 17-13).15,20 A high
exceeds 10 L/min, the effect of the ET might not be significant (see Fig. 17-13).15,20 A high 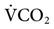 is seldom required for an intubated patient who is breathing spontaneously; otherwise, the patient would receive ventilatory support.
is seldom required for an intubated patient who is breathing spontaneously; otherwise, the patient would receive ventilatory support.
When an appropriate-size ET is used, the imposed WOB may not be any greater through the tube than it is through the upper airway once the patient has been extubated.38 In some studies, ATC was found to be the equivalent of pressure support (PS) (5 cm H2O) and CPAP (5 cm H2O) in reducing WOB.25,39 In some cases ATC might give the false impression that the patient is ready to be extubated, when in fact the person is dependent on the support supplied by ATC, even though it is minimal.32 Also, depending on the ventilator model, ATC may not provide sufficient compensation for WOB imposed by the ET.32,33
Summary of Automatic Tube Compensation
Automatic tube compensation may reduce resistive WOB and increase patient comfort, depending on the ventilator and the artificial airway used. Additional studies are required, however, to determine whether ATC provides all the benefits for which it was designed. Despite the concerns previously mentioned, ATC may represent another method that can be used successfully to extubate patients who are difficult to wean.40,41
Volume-Targeted Pressure-Support Ventilation
Volume-targeted PSV was briefly described in Chapter 6. This mode, which is called volume support (VS) ventilation, on the Servoi ventilator (Maquet Inc.,Wayne, N.J.) is basically PSV with a volume target. Volume-targeted PSV provides a set VT while using PSV criteria (patient triggered, pressure targeted, flow cycled). Although volume-targeted PSV has the advantage of maintaining a target volume, its value in weaning patients from mechanical ventilatory support has not been established.42 Furthermore, several drawbacks have been noted with using volume-targeted PSV (see Chapter 17 section on closed-loop ventilation asynchrony).
Automode and Variable Pressure Support/Variable Pressure Control
Automode is available on the Servo 300 and Servoi ventilators. A similar mode, called variable pressure support/variable pressure control (VPS/VPC), is available on the Venturi ventilator (Cardiopulmonary Corp, Milford, Conn.).
When automode (or VPS/VPC) is activated, the ventilator can switch from a time-triggered mandatory breath to a patient-triggered support breath. For example, if a postoperative patient is still recovering from the effects of anesthesia and the ventilator operator has selected volume-controlled continuous mandatory ventilation (VC-CMV) with automode as the operating mode, all breaths are mandatory (time triggered, volume limited, and time cycled). If the patient begins to trigger breaths, the ventilator switches to VS (patient triggered, pressure limited, and flow cycled with a volume target) and remains in this mode as long as the patient is breathing spontaneously. If the patient becomes apneic again or if no patient effort is detected within a certain period, the ventilator switches back to the support mode (VC-CMV). Automode also can be set to switch from pressure control ventilation (PC-CMV) to PSV and from pressure-regulated volume control (PRVC) to VS.
Automode has been shown to be an effective weaning technique that typically requires fewer ventilator manipulations than other techniques (e.g., SIMV).43,44 Additional clinical studies are needed to evaluate more completely the performance of Automode as a weaning technique.34
Mandatory Minute Ventilation
Mandatory minute ventilation (MMV) was first described in 1977 by Hewlett et al.45 MMV is a closed-loop system in which the ventilator monitors set parameters and makes adjustments accordingly. With traditional weaning methods (e.g., SIMV and PSV), a constant level of ventilation is not guaranteed. With MMV the ventilator automatically increases the level of support if the patient’s spontaneous ventilation decreases, thus maintaining a consistent minimum 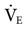 . Patients who regain the ability to breathe spontaneously can increase their own
. Patients who regain the ability to breathe spontaneously can increase their own 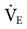 , and the machine automatically lowers support without the clinician having to change any specific ventilator settings. Some of the potential benefits of MMV are listed in Box 20-3.45,46 MMV may be effective as SIMV for weaning patients and less demanding on the ICU staff.47
, and the machine automatically lowers support without the clinician having to change any specific ventilator settings. Some of the potential benefits of MMV are listed in Box 20-3.45,46 MMV may be effective as SIMV for weaning patients and less demanding on the ICU staff.47
The mechanism for providing MMV varies. The ventilator can either adjust the frequency or the VT to maintain the desired 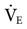 . For example, the Dräger E-4 increases the delivered respiratory rate if a patient’s spontaneous ventilation drops below a desired monitored level. The Hamilton Veolar adjusts the pressure level to increase or decrease VT and adjust
. For example, the Dräger E-4 increases the delivered respiratory rate if a patient’s spontaneous ventilation drops below a desired monitored level. The Hamilton Veolar adjusts the pressure level to increase or decrease VT and adjust 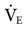 delivery.
delivery.
A potential problem with MMV is that a rapid, shallow respiratory pattern may meet the preset 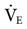 , but it leads to increased dead space ventilation. This pattern can result from a decrease in compliance associated with pulmonary congestion, pulmonary edema, pleural effusion, fibrosis, atelectasis, and pneumonia. It can also be associated with abdominal distension or a decrease in ventilatory muscle strength. As a precaution, the high f and low VT alarms must be set appropriately.
, but it leads to increased dead space ventilation. This pattern can result from a decrease in compliance associated with pulmonary congestion, pulmonary edema, pleural effusion, fibrosis, atelectasis, and pneumonia. It can also be associated with abdominal distension or a decrease in ventilatory muscle strength. As a precaution, the high f and low VT alarms must be set appropriately.
Although few clinical studies address the use of MMV as a weaning technique, several guidelines should be kept in mind. The target 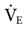 is set slightly below the patient’s total
is set slightly below the patient’s total 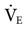 , which includes both mandatory and spontaneous breaths. If a patient is on CMV and is neither hypocapnic or alkalotic, the
, which includes both mandatory and spontaneous breaths. If a patient is on CMV and is neither hypocapnic or alkalotic, the 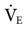 can be appropriately set at 80% of the patient’s previous level. A lower level (i.e., ≤75%) may be adequate if the patient is slightly alkalotic or hypocapnic. For patients on SIMV, setting the
can be appropriately set at 80% of the patient’s previous level. A lower level (i.e., ≤75%) may be adequate if the patient is slightly alkalotic or hypocapnic. For patients on SIMV, setting the 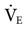 at 90% of the mandatory SIMV value may be adequate.45,46
at 90% of the mandatory SIMV value may be adequate.45,46
It is important to recognize that there are a number of problems that can occur when using MMV, including the development of rapid, shallow breathing patterns, breath stacking (auto-PEEP), delivery of very high VT (inappropriately set upper pressure limit), increased dead space ventilation, and inappropriate settings resulting from clinician misunderstanding or misapplication of the mode. Clinicians must be aware of the potential consequences of changing dead space, carbon dioxide (CO2) production, and patient WOB. The patient’s breathing pattern and gas exchange can vary and therefore must be monitored regularly. Although MMV has been available for three decades, research data on the effectiveness of this mode are still lacking.
Adaptive Support Ventilation
Adaptive support ventilation (ASV) is available on the Hamilton G5 ventilator (Hamilton Medical, Bonaduz, Switzerland). Both ASV and its predecessor, adaptive lung ventilation (ALV), were designed to make automatic adjustments from the time ventilation was initiated until ventilation could be discontinued. The technical aspects have been described elsewhere.48,49 ASV is a patient-centered method of closed-loop mechanical ventilation that increases or decreases ventilatory support based on monitored patient parameters.
Basically, ASV provides pressure-limited breaths that target a volume and rate. The rate and VT are selected by the ventilator’s algorithm to provide the minimum WOB for the patient.50 ASV monitors variables—such as pressure, flow, inspiratory and expiratory time, compliance, resistance, and time constants—to ensure delivery of an acceptable 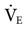 based on practitioner settings. These settings include the patient’s ideal body weight, the high pressure limit, PEEP, FIO2, rise time, flow cycle, and percentage of predicted
based on practitioner settings. These settings include the patient’s ideal body weight, the high pressure limit, PEEP, FIO2, rise time, flow cycle, and percentage of predicted 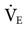 desired. ASV is designed to minimize WOB and auto-PEEP and is capable of ventilator management in a variety of patient situations, including during thoracic surgery.51 ASV also has been studied during ventilator discontinuation,52–54 and it appears to be as safe and effective as traditional methods of weaning and may find increased use in the future.34
desired. ASV is designed to minimize WOB and auto-PEEP and is capable of ventilator management in a variety of patient situations, including during thoracic surgery.51 ASV also has been studied during ventilator discontinuation,52–54 and it appears to be as safe and effective as traditional methods of weaning and may find increased use in the future.34
Artificial Intelligence Systems
This method of weaning patients from ventilatory support relies on artificial intelligence technology. Presently, the only commercial system that is available for clinical use is the SmartCare/PS system, which is offered on the Dräger XL ventilator. The SmartCare/PS system uses predetermined ranges for V, f, and PETCO2 to adjust the inspiratory pressure automatically to maintain the patient in a respiratory “zone of comfort.”23,55 The patient’s readiness for extubation is based on achieving the predefined lowest level of inspiratory pressure. Several factors can affect the lowest level of inspiratory pressure, including the type of artificial airway (i.e., ET versus tracheostomy tube), the type of humidifier (i.e., HME versus heated humidifier), and the use of automatic tube compensation.
Once the lowest level of inspiratory pressure is achieved, a period of observation is initiated during which the patient’s VT, fb, and PETCO2 are monitored. If the patient successfully passes this modified spontaneous breathing trial (SBT), the system automatically displays a message suggesting that the clinician should consider separating the patient from the ventilator.23 It has been suggested that this approach to weaning may be a viable alternative because it reduces the duration of mechanical ventilation and ICU stays.56,57 Additional clinical trials will be required to better define the use of these systems.
Evidence-Based Weaning
The challenge of successfully liberating a patient from a ventilator has been the subject of considerable debate. Solid evidence on identifying a patient’s readiness to wean and ways to accomplish this task remain points of intense discussion among clinicians. In 1999 the federal Agency for Healthcare Policy and Research (AHCPR) asked the McMaster University Outcomes Research Unit to do a comprehensive review of the literature on ventilator withdrawal issues to establish the evidence on which ventilator weaning is based.58 Using the results of the literature review, a task force of the ACCP, the SCCM, and the AARC created evidence-based guidelines for ventilator weaning for patients requiring more than 24 hours of ventilation.59 These guidelines (Box 20-4) form the basis for much of the material presented in the remainder of this chapter.1
Evaluation of Clinical Criteria for Weaning
Three key points have evolved as criteria for weaning:
Recommendation 1: Pathology of Ventilator Dependence
Although it is often overlooked, the primary pathological event that led to initiation of ventilatory support must be corrected. The clinician must determine whether this disease process or condition has improved or been reversed. If not, weaning attempts are unlikely to be successful.59
The ACCP/SCCM/AARC task force’s first recommendation is that a search for all the causes that may be contributing to ventilator dependence should be undertaken for patients who require mechanical ventilation for longer than 24 hours. This recommendation is especially important for patients for whom attempts to be weaned from the ventilator have failed. Reversing all possible ventilator and nonventilator issues is a key part of the ventilator discontinuation process. Box 20-5 provides a summary of factors that must be evaluated to determine a patient’s readiness for ventilator disconnection.6,60,61 Even if the disease process or condition that led to mechanical ventilation has improved or has been reversed, other factors must be considered, such as the patient’s overall medical condition, a physical assessment of cardiopulmonary reserve and WOB, and the patient’s psychological readiness (see Box 20-4).
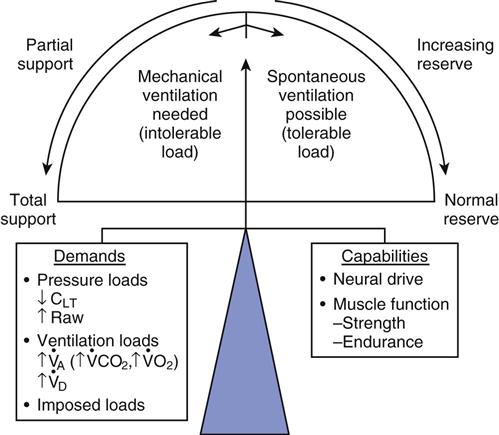
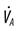 , alveolar ventilation;
, alveolar ventilation; 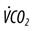 , carbon dioxide production;
, carbon dioxide production; 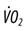 , oxygen consumption;
, oxygen consumption; 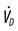 , dead space ventilation.
, dead space ventilation.