Ventilator-Associated Pneumonia
Learning Objectives
On completion of this chapter, the reader will be able to do the following:
1 Define ventilator-associated pneumonia (VAP) and hospital-acquired pneumonia (HAP).
2 Differentiate between early-onset VAP and late-onset VAP and describe the overall incidence of VAP.
3 Discuss the prognosis, including morbidity and mortality rates, for patients diagnosed with VAP.
4 Identify the most common pathogenic microorganisms associated with VAP.
6 Describe the sequence of events that are typically associated with the pathogenesis of VAP.
Key Terms
• Bronchial alveolar lavage
• Clinical Pulmonary Infection Score
• Deescalation
• Early-onset pneumonia
• Fiberoptic bronchoscopy
• Gastroprotective agents
• Health care–associated pneumonia
• Hospital-acquired pneumonia
• Kinetic therapy
• Late-onset pneumonia
• Multidrug-resistant microorganisms
• Nosocomial infection
• Polymicrobial
• Protected specimen brush
• Superinfections
• Ventilator-associated pneumonia
Ventilator-associated pneumonia (VAP) is defined as pneumonia that develops 48 hours after a patient has been placed on mechanical ventilation. It is an important subset of hospital-acquired pneumonia (HAP), which is pneumonia that occurs 48 hours or longer after admission to the hospital and results from an infection that was not incubating at the time of admission. HAP is differentiated from healthcare–associated pneumonia (HCAP), which afflicts patients who have resided in a long-term care facility or received acute care in an acute-care hospital for a specified time before developing pneumonia (i.e., 2 or more days within 90 days of the infection) (Key Point 14-1).
Most often VAP is caused by bacterial infections, but it can be caused by fungal infections or may be associated with viral epidemics (e.g., SARS [severe acute respiratory syndrome]) (Box 14-1). VAP that develops between 48 and 72 hours following tracheal intubation is usually classified as early-onset pneumonia, whereas pneumonia that develops later than 72 hours is considered late-onset pneumonia.1,2
Despite major advances in the management of ventilator-dependent patients, VAP continues to complicate the course of treatment of a significant number of patients receiving invasive mechanical ventilation.3 Development of VAP is associated with prolonged hospital stays, increased health care cost, and mortality rates that range from 25% to 50%. 3–7
Guidelines for the management of patients with VAP focus on early diagnosis, appropriate antibiotic treatment, and various strategies to prevent the transmission of pathogenic organisms to patients receiving mechanical ventilation. Although there has been considerable debate among clinicians regarding the most effective means of diagnosing and treating VAP, it is agreed that successful management of VAP requires early diagnosis and appropriate use of antibiotic therapy to avoid the emergence of multidrug-resistant (MDR) microorganisms (Key Point 14-2). Effective infection-control procedures and surveillance techniques are also necessary to prevent the transmission of nosocomial infections. Careful handwashing with antimicrobial agents, proper disinfection and sterilization of respiratory therapy equipment along with the adherence to standard and disease-specific precautions, and implementation of clinical protocols, such as “VAP bundles,” can significantly reduce the incidence of VAP.8
It is beyond the scope of this text to review every clinical study that has been conducted on VAP. A list of selected articles is provided at the end of the chapter for readers interested in further detail on specific studies about the management of patients with VAP, HAP, and HCAP.
Epidemiology
Ventilator-associated pneumonia is the most common nosocomial infection encountered in the intensive care unit (ICU).5 The highest risk for the development of VAP occurs early in the course of the hospital stay. Cook and colleagues estimated that the risk of development of VAP is about 3% per day during the first 5 days of receiving mechanical ventilation, 2% per day for days 5 through 10, and 1% thereafter.9
The incidence of VAP ranges from 8% to 28% for all intubated patients.3,5,10 Clinical studies have consistently demonstrated that critically ill patients with VAP have significantly higher mortality rates than mechanically ventilated patients without pneumonia. The overall attributable mortality rate for VAP ranges from 5% to 48%, depending on the infecting organism(s), the presence of underlying disease, and prior antimicrobial therapy.3,11–15
The prognosis for patients with early-onset VAP is generally better than those who develop pneumonia later in the course of treatment.9 The reason for the better prognosis for early-onset VAP is related to the fact that these patients are typically infected with antibiotic-sensitive bacteria, whereas patients with late-onset VAP (i.e., longer than 5 days) are more likely to be infected with MDR pathogens.
Causes and Risk Factors
Ventilator-associated pneumonia has been linked to the aspiration of oropharyngeal secretions and esophageal/gastric contents, direct inoculation of infectious material into the trachea and lungs during endotracheal intubation, inhalation of infected aerosols, embolization of biofilm that can be found in the endotracheal tubes (ETs) of patients receiving prolonged mechanical ventilation, exogenous penetration from the pleural space, and the hematogenous spread of extrapulmonary infections to the lung.5,16
Box 14-1 lists the most prevalent aerobic gram-negative and gram-positive bacteria that have been identified as potential pathogens responsible for VAP. Historically, aerobic gram-negative bacilli have accounted for nearly 60% of all VAP infections with Pseudomonas aeruginosa, Klebisella pneumonia, Escherichia coli, and Acinetobacter occurring at the highest frequency17 (Key Point 14-3). More recent studies have shown that gram-positive bacteria are becomingly increasingly more common in VAP, with methicillin-resistant Staphylococcus aureus (MRSA) being the predominant gram-positive organism isolated.3,7,18 Polymicrobial infections (i.e., infection by multiple pathogenic microorganisms) constitute nearly 50% of all VAP infections, although pathogenic anaerobic infections are not typically found in these mixed-type infections.3
Various independent factors contribute to the development of VAP or may increase the frequency of complications in these patients. Box 14-2 lists several host-related factors and therapeutic interventions that have been identified as risk factors for VAP. Notice that these factors are generally related to the characteristics of the patient populations affected (e.g., age of the patient, diagnosis at admission, severity of the illness, presence of comorbidities), as well as the impact of using various pharmacologic interventions and respiratory therapy modalities in the treatment of ventilator-dependent patients.
Older patients are at greater risk for developing VAP than are younger patients. Patients treated for trauma, burns, multiorgan failure, or impaired levels of consciousness typically have the highest risk for development of VAP. The presence of comorbidities may actually predispose patients to infections with specific organisms. For example, patients with chronic obstructive pulmonary disease, or COPD, have an increased risk for H. influenza, S. pneumonia, and Moraxella catarrhalis, whereas cystic fibrosis patients are susceptible to P. aeruginosa and S. aureus infections.3 MRSA is particularly prevalent in patients with diabetes, head trauma, and those who have been hospitalized for prolonged periods in the ICU.7 VAP is also recognized as a major complication of acute respiratory distress syndrome (ARDS). It has been estimated that 35% to 70% of ARDS patients develop pneumonia, which can lead to sepsis and multiple organ failure. The mortality rate for ARDS patients with VAP is significantly higher than patients without VAP.3,19
Therapeutic interventions are generally categorized as pharmacologic and nonpharmacologic. Examples of pharmacologic interventions that can lead to the development of VAP or complicate the course of treatment for these patients include concurrent steroid therapy, inappropriate antimicrobial therapy, overuse of sedatives and paralytics for mechanically ventilated patients, and the use of type 2 (H2) histamine antagonists and gastroprotective agents, such as antacids.
Inappropriate use of antibiotics in the hospital setting is particularly troublesome because it has been associated with the selection of MDR pathogens3,18,20 (Key Point 14-4). Prolonged antibiotic administration to ICU patients for a primary infection may favor selection and subsequent colonization with resistant pathogens responsible for superinfections.3 This is an important issue for patients with late-onset VAP because as mentioned previously, these patients are at a higher risk for being infected with MDR pathogens. Imprudent use of sedatives and paralytics can also increase the incidence of VAP by impairing the patient’s level of consciousness, which can ultimately blunt the patient’s cough reflex and increase the chances of aspiration. Box 14-3 lists the most common risk factors for MDR infections.
Nonpharmacologic interventions that are associated with the increased risk of VAP include the need for an ET or tracheostomy tube during ventilation; routine care of ventilator circuits, humidifiers, and nebulizers; and the use of respirometers, reusable electronic ventilator probes and sensors, bronchoscopes, and endoscopes.5 The most important of these nonpharmacologic factors that has been found to be associated with VAP is the use of an ET or tracheostomy during mechanical ventilation. The incidence of VAP is 6 to 21-fold higher in patients who are intubated receiving mechanical ventilation compared with the incidence in patients receiving noninvasive mechanical ventilation. This has led some clinicians to suggest that “endotracheal intubation-associated pneumonia” might be a more appropriate name for this type of pneumonia.
Respiratory therapy equipment has long been implicated as a source of nosocomial infections. Indeed, epidemics of HAP and VAP are most often associated with contamination of respiratory therapy equipment, bronchoscopes, and endoscopes. Instituting stringent infection-control procedures can reduce the incidence of nosocomial infections in hospitals and other health care facilities; however, ensuring that all of the clinical staff members adhere to the prescribed infection-control policies remains a formidable task. Surveillance of ICU patients at high risk for bacterial pneumonia can also be an important part of determining trends and identifying outbreaks.21 Additional details on various nonpharmacologic strategies that can be used to reduce the incidence of VAP are presented later in this chapter.
Pathogenesis of Ventilator-Associated Pneumonia
The pathogenesis of VAP most often involves colonization of the aerodigestive tract with pathogenic bacteria, aspiration of contaminated secretions into the lower airways, followed by colonization of the normally sterile lower airways and lung parenchyma with these infectious microrganisms.15 The upper airways of healthy individuals typically contain nonpathogenic bacteria, such as viridans group of streptococci, Haemophilus spp., and anaerobes.5 Aerobic gram-negative bacilli, most notably virulent forms of P. aeruginosa and Acinetobacter, are rarely found in the respiratory tract of healthy individuals because of anatomic barriers, the cough reflex, mucociliary clearance mechanisms, and innate cellular and humoral immune factors (e.g., leukocytes, immunoglobulins).
During critical illnesses, particularly in patients with an endotracheal tube and receiving mechanical ventilation, there is a dramatic shift in the flora of the oropharyngeal tract to gram-negative bacilli and S. aureus.5,7 This shift in flora may be attributed to a number of factors that compromise host defenses mechanisms, including comorbidities, malnutrition, reduced levels of mucosal immunoglobulin A, increased production of proteases, exposed and denuded mucous membranes, elevated airway pH, and an increased number of airway receptors for bacteria as a result of acute illness and prior antimicrobial use.5,22–24 Aspiration of the contaminated oropharyngeal secretions and, in some cases, gastroesophageal contents can occur because the patient is unable to protect the lower airways. Impaired level of consciousness, gastroesophageal reflux, a blunted gag reflex, and abnormal swallowing can all contribute to the risk of aspiration.15 After these offending organisms penetrate and colonize the lower airways, they can overwhelm already compromised pulmonary cellular and humoral immune defense mechanisms and eventually lead to VAP.15
Diagnosis of Ventilator-Associated Pneumonia
The lack of a gold standard for the diagnosis of VAP has caused considerable debate among clinicians.25,26 It has been suggested that clinical criteria involving patient symptoms and signs, chest radiographs, and baseline hematologic studies can be effective for starting empiric antibiotic therapy; however, simply relying on clinical findings to guide therapeutic interventions may result in a failure to accurately diagnose VAP and lead to inappropriate antibiotic therapy if the infection is polymicrobial in origin or if a drug-resistant organism is present. Current recommendations from the American Thoracic Society (ATS) and the Infectious Diseases Society of America (IDSA) suggest that invasive microbiologic procedures, such as quantitative cultures of lower respiratory secretions obtained by bronchial alveolar lavage (BAL) or protected specimen brush (PSB) procedure are often necessary to ensure effective treatment of patients with VAP.7
Clinical Diagnosis
Ventilator-associated pneumonia should be suspected when a mechanically ventilated patient demonstrates radiographic evidence of new or persistent infiltrates for longer than 48 hours and at least two of the following findings: fever, leukocytosis, and purulent tracheobronchial secretions. Table 14-1 provides a list of clinical criteria that can be used in the clinical diagnosis of VAP. It is worth mentioning that fever and the presence of pulmonary infiltrates on chest radiographs are nonspecific findings that can be associated with numerous other conditions, including chemical and radiation pneumonitis, atelectasis, pulmonary embolism and infarction, lung contusion, ARDS, and drug or hypersensitivity reactions.16,26
TABLE 14-1
Clinical Criteria Used in the Diagnosis of Ventilator-Assisted Pneumonia (VAP)27,29
| POINTS | |||
| Variables | 0 | 1 | 2 |
| Temperature, °C | ≥36.1 to ≤38.4 | ≥38.5 to ≤38.9 | ≥39 to ≤36 |
| WBC count, µL | ≥4,000 to ≤11,000 | <4,000 to >11,000 | |
| Secretions: | Absent | Present, nonpurulent | Present, purulent |
| PaO2/FIO2 | >240 or ARDS | ≤240 and no ARDS | |
| Chest radiography | No infiltrate | Diffuse or patchy infiltrate | Localized infiltrate |
| Microbiology | No or light growth | Moderate or heavy growth; add 1 point for same organism on Gram stain | |
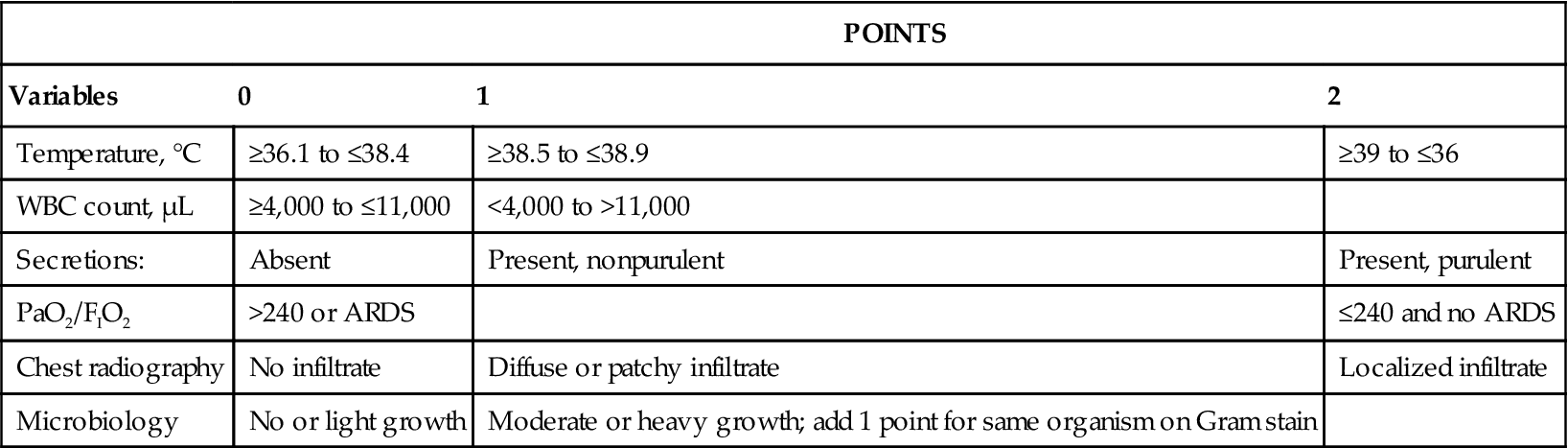
ARDS, Acute respiratory distress syndrome; PaO2, arterial oxygen pressure; WBC, white blood cell.
From Porzecanski I, Bowton DL: Diagnosis and treatment of ventilator-associated pneumonia, Chest 130:597-604, 2006.
Some clinicians emphasize certain findings over others using a “weighted” approach to clinical diagnosis.26 The Clinical Pulmonary Infection Score (CPIS) is an example of this type of approach. The CPIS includes six clinical assessments with each item given a score of 0 to 2 points (see Table 14-1). The assessment criteria include fever, leukocyte count, quantity and purulence of tracheal secretions, oxygenation status, the type of radiographic abnormality, and results of a tracheal aspirate culture and Gram stain. (Note that a modified CPIS in which the endotracheal aspirate culture and Gram stain results are excluded is also available. In this case, the score will range from 0 to 10 instead of 0 to 12).26–29 When all six criteria are used, a score greater than 6 is considered evidence of the presence of VAP.27 It is generally accepted that measurements of CPIS should be performed at the beginning of antibiotic therapy and after 2 to 3 days to reevaluate the effectiveness of the treatment course. Although some investigators have found considerable interobserver variability and a lack of specificity to guide antibiotic therapy, a case can be made that measurement of the CPIS may reduce the mortality rate associated with VAP.28,29 The measurement of CPIS may also provide information that can allow the clinician to aggressively treat patients with VAP while limiting the course of antibiotic therapy and thus controlling for the development bacterial resistance26 (Case Study 14-1).



