Fig. 9.1
Range and possible yield of mediastinal dissection by the means of VAMLA (central, blue ellipse) and additional extended mediastinoscopy (central, green ellipse), compared to right and left lateral accesses by VATS or thoracotomy (grey ellipses). VAMLA is superior especially to left-sided VATS and open lymphadenectomy. (Adapted from Mountain CF et al. [50])
9.2 Historical Note
During the 1990s, video cameras became popular in many fields including surgical endoscopy. Video-mediastinoscopy has been suggested by Toni Lerut in 1987 [4], and commercially available tubular video-mediastinoscopes appeared several years later. The technical change of the imaging system led to improved visibility, facilitating the performance and teaching of the procedure as well as the storage of visual information [5]. Nevertheless, as the design of the scope itself remained unchanged, video-mediastinoscopy was employed and executed more or less like it had been introduced by Carlens in 1959 [6]: as a method for inspection and biopsy, with similar results [7, 8]. In contrast to laparoscopic or thoracoscopic minimally invasive surgery, the mediastinal working space is neither preformed nor ample; moreover, the cervical approach precludes the use of multiple ports. It was the concept and realisation of a two-bladed spreadable video-mediastinoscope by Linder and Dahan (Figs. 9.2 and 9.3) that provided a wide operation field, thus facilitating bimanual manoeuvres. A prototype of the Linder-Dahan mediastinoscope was put into action by Martin Huertgen and Albert Linder at the Schillerhöhe Hospital, Stuttgart-Gerlingen, Germany. Accumulating experience with the new machine, they made two observations: first, the removal of mediastinal lymph nodes was safer and easier than their biopsy, and second, the complete removal of mediastinal lymphatic tissue was feasible. Huertgen developed and evaluated a technique of minimally invasive mediastinal dissection, video-assisted mediastinoscopic lymphadenectomy, first published using the acronym VAMLA in 2002 [9]. Concurrently, neoadjuvant treatment of stage III [10, 11] and VATS lobectomy for stage I lung carcinoma [12–14] were introduced, both highlighting unresolved questions concerning mediastinal staging and dissection [15, 16].

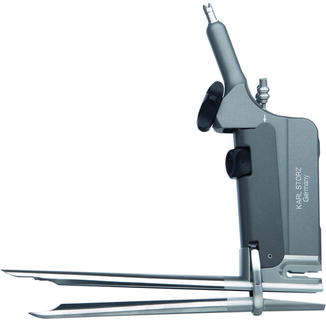

Fig. 9.2
The Linder-Dahan video-mediastinoscope is a crossbred of conventional mediastinoscope, spreadable laryngoscope, and video-mediastinoscope. Its blades are spread open up to 32 mm by turning screw (a). Lowering the lower blade by turning the spacer (b) allows additional 20 mm of operation field. The tip of the upper blade (c) is designed to keep surrounding mediastinal structures, e.g. fatty tissue or the pulmonary artery, out of the operation field. It accommodates light, optic, continuous suction, and irrigation

Fig. 9.3
The second generation: Linder-Huertgen mediastinoscope with further spreadable blades, modular integration of several technical parts (camera, fixation and adjusting screws, inside tubing), and different sizes available
9.3 Indications
At present, performing VAMLA for mediastinal staging and dissection is an individual decision in every respect. To develop an institutional decision, the known arguments in favour of the VAMLA approach to mediastinal staging and dissection should be considered [17–19]: the high staging accuracy, the avoidance of re-mediastinoscopies after neoadjuvant treatment, the improvement of mediastinal dissection in combination with left-sided and VATS lung resections, the unity of staging and dissection within one procedure, and the possible combination with extended mediastinoscopy, mediastinoscopic ultrasound, or right-sided video-thoracoscopy within the same procedure. To come to an appropriate individual indication [17], the probability of mediastinal involvement, the size and resectability of mediastinal nodes, the laterality of the primary, and the ability of the patient to withstand invasive mediastinal staging and possibly multimodality treatment should be taken into account. Ideally, institutional practice is accompanied by prospective observation and resulting in publication.
New indications are facilitated by the clinical versatility of VAMLA. It appears obvious to combine VAMLA and nonsurgical modalities as radiation therapy [20] and radiofrequency ablation. In selected patients, there might be a role for VAMLA in palliative treatment concepts. The rationale is always the improvement of local mediastinal control in patients unsuitable for lung resection to prevent local complications like bleeding, infection, respiratory insufficiency, and asphyxia, thus maintaining quality of life and a stable clinical state facilitating palliative treatment.
For clinical research, VAMLA offers several possibilities. One field of research is surgical evaluation of other methods of mediastinal staging, in analogy to the evaluation of EUS-FNA already presented [21]. Then, unfortunately, the majority of studies evaluating new therapies or new prognostic factors harbour very low diagnostic certainty with regard to mediastinal staging. The known survival differences between cN and pN stages [22] is well within the range of the differences observed in many oncological trials, suggesting the application of VAMLA to provide accurate staging, and, if surgery is part of the protocol, adequate dissection. Furthermore, the typical surgical sequence of VAMLA and lung resection can serve as a clinical or animal model to study the local tolerance and resorption kinetics of resorbable material [23].
A survey of possible clinical and scientific applications is given in Table 9.1.
Table 9.1
Suggested indications for VAMLA
Context | Application | Objective |
|---|---|---|
Research | Evaluation of new prognostic factors and therapies | Accurate mediastinal staging, a prerequisite for comparable results |
Evaluation of mediastinal staging techniques | Surgical assessment of accuracy | |
Evaluation of implants, e.g. resorbable haemostats | Local tolerance | |
Resorption kinetics | ||
Surgery | Left-sided tumours | Complete mediastinal dissection |
VATS lobectomy/segmentectomy | ||
Oncology | Minor N2/3: mediastinal staging/clearance before neoadjuvant treatment | More neoadjuvant treatment |
Apparent FNA-proved N2/3: mediastinal restaging after neoadjuvant therapy | No re-mediastinoscopies | |
Identification of false-negative/false-positive scans, e.g. PET-CT | No under-/overtreatment | |
Radiation oncology | Involved field radiation | Improved mediastinal control |
Combination with SBRT/RFA | ||
Additional staging procedures | Extended mediastinoscopy | Suspicious para- and subaortic lymph nodes |
Mediastinal ultrasound (MUS) | Resectability of central tumours | |
Right-sided video-thoracoscopy | Confirming mesothelioma, pleural carcinosis |
9.4 Contraindications
VAMLA is contraindicated in the presence of extensive mediastinal disease.
9.5 Technique [9, 24, 25]
The two principles of VAMLA are a minimally invasive access similar to conventional mediastinoscopy and the compartmental en bloc resection of the entire mediastinal adipose tissue guided by anatomical landmarks similar to open systematic nodal dissection [26].
To overcome this evident contradiction, VAMLA requires a bimanual dissection technique, dependent on a spreadable video-mediastinoscope (Figs. 9.2 and 9.3) and a few dedicated instruments.
Basic dissection technique consists of two steps: first, applying tension on a dissection plane and then dividing. The dissection plane is most often exposed by manoeuvring the specimen. Moving the specimen inwards, away from the optic, is advantageous to maintain a proper view. The specimen is easily manoeuvred by a large grasper with blunt fenestrated claws. Firm closure of the instrument is almost never required and should be avoided, as it will cause tissue fragmentation. Division of connective tissue planes and fibres requires blunt and sharp dissection. The suction-coagulation device provides both modalities and does no harm, as long as the coagulation as well as cutting current is kept within a range that avoids carbonisation (soft-coagulation mode). Prerequisite for any dissection is a clear vision. For this purpose, both the suction-coagulation device as well as the suction channel incorporated into the mediastinoscope should be kept in continuous action throughout the procedure. Irrigation is very helpful to clear not only the dissection site but also the tip of the optic. The latter requires forceful flushing of 5–10 ml aliquots, and sometimes additional cleansing by a small sponge stick.
To perform VAMLA, the mediastinum is divided in three compartments according to anatomy and practical surgical reason, which are dissected sequentially. Interestingly, this very kind of compartmentation was reproduced by the concept of mediastinal zones and the IASCL lymph node map [27].
Standard dissection was defined to include all lymph node stations within the range of conventional mediastinoscopy. It includes the subcarinal compartment containing the subcarinal lymph nodes; the right compartment containing the pretracheal, right paratracheal, and tracheobronchial nodes; and the left compartment containing the left paratracheal and tracheobronchial nodes, thus providing bilateral clearance of the nodal stations 2, 4, and 7. As the range is not limited by this definition but by the dimensions of the video-mediastinoscope in relation to the patient, it is possible to include supraclavicular, prevascular, paraesophageal, hilar, and interlobar nodes (stations 1, 3a, 8, 10, and 11R) and, via extended mediastinoscopy [28], the para- and subaortic nodes (station 5 and 6 L), if required within an individual clinical context.
Similar to conventional mediastinoscopy, VAMLA starts with a small jugular access to the cervical trachea and digital mobilisation of the airway and the innominate artery (Fig. 9.4). The closed mediastinoscope is inserted to expose and separate the main bifurcation and pulmonary artery. The scope is placed in between, loading the artery on its upper blade, and then fixed and spread to display the subcarinal compartment and to excise the subcarinal tissue en bloc, following the medial borders of both the main bronchi and the anterior oesophagus wall (Fig. 9.5a). Then, the scope is retracted to the innominate artery and directed to the right side. Pushing the pretracheal and right paratracheal fat pad downwards, the parietal pleura and superior vena cava are exposed, and the fat part is removed en bloc down to the azygos vein and the right main bronchus (Fig. 9.5b). Finally, on the left side, the recurrent nerve is visualised, and the pretracheal fascia is divided between the nerve and the left tracheobronchial angle. To protect the nerve as well as its function, careful systematic dissection and removal of all visible lymph nodes are preferable to en bloc resection of the left paratracheal and tracheobronchial adipose tissue (Fig. 9.5c). For the same reason, electric current is not appropriate on the left side, and haemostasis is relying on clips, temporary compression, or resorbable haemostats [23]. This should also be considered in the supravascular zone, where both recurrent nerves are potentially at risk.
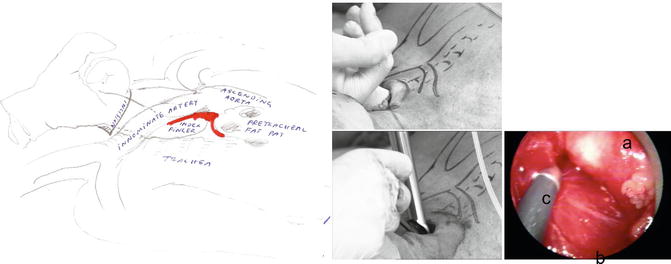
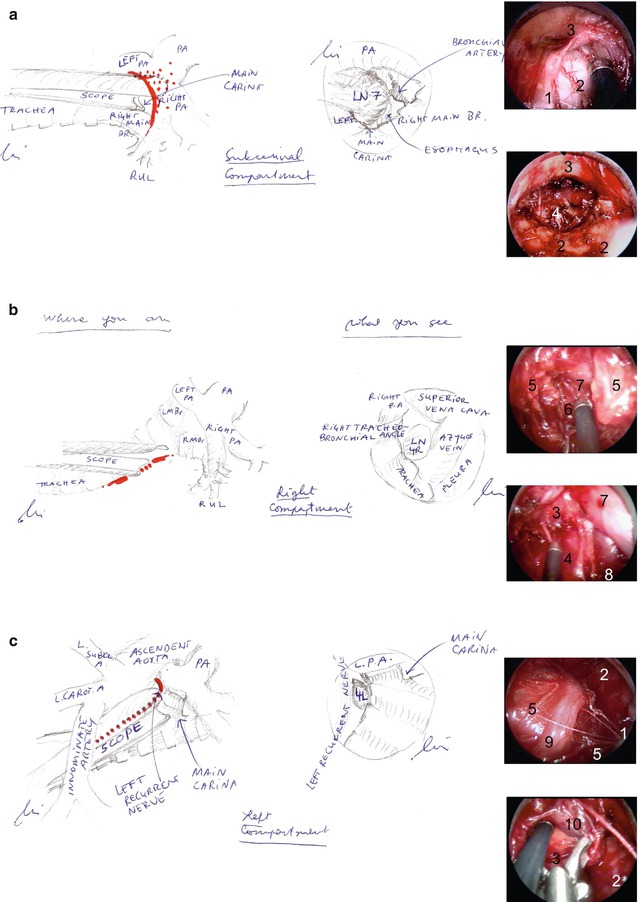

Fig. 9.4
Digital mobilisation of the innominate artery and pretracheal fat pad (left) before introducing the scope and visualising the artery (right). Legend: a innominate artery, b trachea, c suction-coagulation device

Fig. 9.5
Dissection of the subcarinal (a), the right (b) and left (c) compartment, illustrated by drawings showing the position of the scope (left) and the view through the scope (centre). On the right, screenshots of each situs are shown at the beginning (above) and the end (below) of the dissection. Legend: 1 trachea, 2 main bronchus, 3 pulmonary artery, 4 oesophagus, 5 pre- and paratracheal adipose tissue, 6 parietal pleura, 7 superior vena cava, 8 azygos vein, 9 left recurrent nerve, 10 tracheobronchial lymph node
Minor haemorrhage is best prevented by clipping the regularly appearing bronchial artery branches crossing the subcarinal space and the small veins draining into the vena cava. Major haemorrhage is very unlikely, if the pulmonary artery is adequately mobilised before the scope is spread open. Precautionary measures to minimise the inherent risk of tumour spillage are important and include the careful handling of the specimen, cutting by electrocautery, deliberate irrigation, and respecting advanced mediastinal disease as a contraindication.
9.6 Results
The first description of the method compared the mediastinal yield of 40 complete VAMLA dissections to 80 cases of open mediastinal dissection from the same institution. The weight of the VAMLA specimen was significantly higher and containing significantly more lymph nodes (20.7 vs 14.3 nodes) than those of the open surgery group [9]. Investigating 20 VAMLA procedures, Leschber et al. described dissection rates of different mediastinal nodal stations within a range of 92–100 % [29]. Both pilot studies suggest that the yield and radicality of VAMLA is comparable to open mediastinal dissection.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


