Fig. 18.1
Bronchoscopic view of a mid-tracheal neoplasm in a patient who presented with haemoptysis
In an appropriate patient, surgical management requires circumferential tracheal resection and reconstruction approached through a transcervical approach. Details of the procedure are addressed below. Negative margins are paramount; in malignant disease, reduced risk for locoregional recurrence is significantly improved by complete excision (R0) [3, 4]. Depending upon tumour location, an R0 resection may require partial laryngectomy or even complete laryngotracheal resection if there is extensive proximal extension. Techniques for resection of these lesions will be discussed elsewhere.
Contraindications for resection of malignant tracheal tumours include extensive involvement of the airway that precludes primary reconstruction without tension, invasion of mediastinal structures preventing an R0 resection, or the presence of distant metastatic disease. In specific circumstances, palliative resection for symptom control or haemoptysis may be an option. Unresectable patients should be referred to a radiation oncologist for primary radiotherapy. Contraindications for resection of benign tumours include the presence of significant airway inflammation or chronic steroid use (>20 mg prednisone daily). Active use of steroids may reduce healing following resection and increase risk for anastomosis breakdown or restenosis [2]. Other relative contraindications include prior radiation, significant comorbidities precluding tolerance of general anaesthesia, and need for postoperative mechanical ventilation.
18.2.1 Surgical Technique
Transcervical resection of the trachea is performed in the supine position. An inflatable bag is placed underneath the shoulders, allowing for neck extension during the procedure and flexion following reconstruction. The bed is positioned with slight flexion at the hips and knees so the neck and upper sternum are positioned in the horizontal plane.
Airway management requires cooperation between the surgery, nursing, and anaesthesia teams. Spontaneous ventilation with a deep level of anaesthesia is most successful for induction. Rigid bronchoscopy allows for visualization of the lesion and, in the case of a tight stenosis, dilation to accommodate a number 6 endotracheal tube (ET). The ET can be placed adjacent to endobronchial tumour, although it is ideally positioned with its tip distal to the lesion. In patients with an existing stoma, oral intubation is preferred and facilitates exposure and excision of the stomal site. The field is prepared and draped so that anaesthesia has access to cross-field ventilation tubing.
A collar incision is made 1 cm above the clavicular heads. An existing tracheostomy stoma may be included in the collar incision or excised separately. The platysma is divided and flaps are created superiorly to the level of the thyroid cartilage and inferiorly to the sternal notch. Skin flaps are spread vertically with Gelpi retractors to enhance exposure, and strap muscles are preserved and retracted laterally. These will be used to cover the anastomosis at case completion. The thyroid isthmus is ligated and divided with clamps, and the pretracheal plane is developed with a combination of blunt and sharp dissection from the thyroid notch to the carina. This maneuver helps mobilize the trachea and reduce anastomotic tension. Dissection should be kept close to the airway, particularly the lateral and posterior aspects of the trachea, to avoid injury to the recurrent laryngeal nerves (RLN). In a “redo” case, cervical anatomy may be distorted, and it is often easier to begin dissection at the lateral aspect of the incision working toward the midline where adhesions are most significant. This technique is also helpful for patients with existing tracheal stomas. The RLNs should not be identified and dissected out; their approximate course in the tracheoesophageal groove should be noted and avoided. This is particularly important in re-operative cases where there often is significant scar tissue around the lateral and posterior aspects of the trachea.
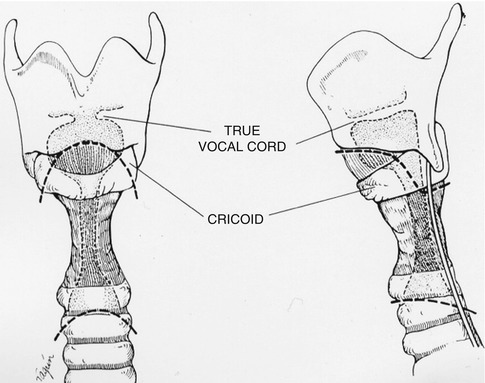
The anterior surface of the trachea is dissected from the cricoid cartilage to the distal resection margin, identified with flexible bronchoscopy and a small-gauge needle passed through the anterior wall of the airway. If additional exposure is necessary, a vertical incision is continued from the midpoint of the collar incision to a point 2 cm below the sternal angle. Underlying tissues are dissected, and the manubrium is divided with a sternal saw using caution to avoid the innominate vein. A small pediatric chest wall retractor can be used to assist with exposure. This maneuver provides adequate visualization of the distal trachea without interference of the great vessels or a complicated dissection. Caution should be exercised in patients with prior cardiac surgery or if there is risk of vascular adherence to the back of the sternum/manubrium. Dissection should be kept close to the trachea, and the innominate artery should not be mobilized. Removal of the native protective tissues of the innominate artery places the vessel at risk for erosion and hemorrhage following reconstruction. If there is concern regarding proximity of the innominate artery to the tracheal anastomosis, placement of strap muscle or thymic tissue as an interposition should be done.
The trachea is dissected circumferentially but only at the level of interest and should not extend beyond 1–2 cm from the resection margins to maintain adequate blood supply to the remaining segments. This technique will significantly reduce the risk of tracheal necrosis [5]. Cross-field ventilation tubing is passed off the field in preparation for tracheal division.
The distal tracheal resection margin is incised circumferentially, and cross-field ventilation is instituted. In benign disease, the amount of normal trachea left behind should be maximized. Clinical judgment is necessary when considering removal of inflammatory segments or excessive lengths of disease that might cause anastomotic tension [5].
The normal distant trachea is inspected for visually clean margins, and 2-0 Vicryl stay sutures are placed vertically in the mid-lateral positions, usually encircling a tracheal ring. The distal end of the specimen is grasped, and dissection is continued proximally to the appropriate point. Dissection may need to extend to or include the anterior cricoid cartilage; the technique for a laryngotracheal resection will be discussed elsewhere. The membranous wall of the trachea is freed from its posterior attachments to the esophagus, and additional traction sutures are placed in the normal proximal trachea after that margin has been established. Special attention regarding the proximal extent of the posterior dissection is necessary: the RLNs are at risk during dissection at the inferior margin of the posterior cricoid plate where the larynx and esophagus are attached [5].
A red rubber catheter is secured to the ET tube with a silk suture. When appropriate, the anaesthesia team should withdraw the ET tube back into the mouth, leaving the red rubber catheter accessible on the field. This catheter serves as a useful guide to reestablish oral ET ventilation during airway reconstruction.
The tracheal specimen is sent for frozen section to confirm negative margins, which is particularly important in cases of ACC where submucosal and perineural spread of the tumour can extend beyond visible disease [6]. The anaesthesia team next deflates the thyroid bag and flexes the neck, while traction sutures are crossed to bring the tracheal margins together ensuring adequate mobilization. Surgical judgment is needed to assess for risk of anastomotic tension and if additional mobilization or a laryngeal release is required. The neck is replaced in extension, and interrupted 4-0 Vicryl sutures are used to create the reconstruction. Of note, the use of Vicryl has markedly reduced the risk of suture line granuloma formation [7].
Initial sutures are placed in the posterior midline of the membranous tracheal wall, from outside in, then back inside out, so that knots are tied outside the lumen. Sutures should be approximately 4 mm apart and 4 mm from the edge of the trachea. Individual sutures are clamped together and then to the bed in a circumferential manner. Each successive suture is laid anterior to the previous one, and sutures are placed from medial to lateral. Cross-field ventilation can intermittently be discontinued and reinserted to improve visualization. Clamps should be secured to the bed in a cephalad to caudad orientation.
Sutures are tied in the reverse order of placement so it is imperative they be organized appropriately. At completion, the oral ET tube is advanced with assistance of the surgeon by gentle traction on the red rubber catheter. Keeping the ET tube free of the reconstructive sutures is imperative.
The thyroid bag is deflated and the neck is flexed and secured with folded blankets. Lateral stay sutures are tied together to reapproximate the tracheal margins; these sutures are intended to reduce tension on the anastomosis and should not be overly tightened causing imbrication. Interrupted Vicryl sutures are tied anterior to posterior from the midline, and sutures are cut as they are tied. When completed the anastomosis is tested by submersion in saline and 30 cm of water pressure generated by continuous forced ventilation. The ET tube cuff should be deflated and an audible air leak or a false-negative test may result. This also allows for assessment of the patency of the repair. Hemostasis is ensured and the strap muscles are reapproximated with interrupted Vicryl sutures. The platysma and skin are closed in two layers over a Jackson-Pratt drain. A heavy braded suture (no. 2) is placed from the submental fold of the chin to the anterior chest wall and tied loosely to serve as a “reminder” to the patient that extension or rotation of the neck should be minimized. Extreme hyperflexion of the neck must be avoided and can be associated with neurologic complications. The patient should be extubated in the operating room, and immediate assessment for resistance to airflow or coarse breath sounds is determined. In cases that involve the larynx, concern for laryngeal edema may justify continued mechanical ventilation for a short postoperative period. Transport to a monitored intensive care unit for observation is standard.
In cases of extended resection, concern for anastomotic tension may prompt performance of a laryngeal release maneuver. Traditional procedures, including the Dedo thyrohyoid release, have fallen out of favor due to risk for abnormal deglutition and postoperative aspiration [8, 9]. We prefer a suprahyoid release, performed through a second short transverse incision directly over the hyoid bone. The hyoid is exposed, and adjacent stylohyoid muscles are identified and divided, as are the muscles attached to the hyoid bone between the digastrics. Finally, the hyoid bone is divided lateral to the lesser cornu and medial to the digastric sling on either side [10]. We typically place an additional drain in this space.
18.2.2 Results
We evaluated the results of 191 patients with ACC or SCC of the trachea that were resected and reconstructed at our institution. Contraindications to surgery included excessive tumour length and the presence of regional or distant metastatic disease. Overall operative mortality was 7.3 % (reduced to 3 % in the last decade of study). Overall 5-year survival and 10-year survival for resected ACC were 52 and 29 %, respectively, and for SCC 39 and 18 %, respectively. Completeness of resection, negative margins, and ACC histology was associated with improved long-term survival [3].
18.3 Secondary Tracheal Neoplasms
Secondary tracheal neoplasms are due to malignant invasion from adjacent structures, most commonly the thyroid, lung, esophagus, or larynx. Hematogenous metastasis from the breast, melanoma, and kidney is less common [11]. If an R0 resection is achievable based on preoperative assessment, it should be considered. Indications and technique will be discussed in further detail.
Resection for palliation, most commonly for shortness of breath or significant obstructive symptoms, may be appropriate in patients who would otherwise tolerate surgery. Palliative procedures, including endobronchial debulking with rigid bronchoscopy, will be discussed elsewhere. Additional therapeutic interventions include laser therapy, radiation, brachytherapy, and endobronchial stents, the use of which has increased significantly in the past decade [12, 13]. Goals of care for palliative procedures include symptomatic relief of airway obstruction and prevention of asphyxiation or bleeding due to tumour progression. This section will focus on tracheal involvement by invasive thyroid carcinoma, the most common secondary tracheal neoplasm encountered.
Transcervical tracheal resection for thyroid carcinoma invading the airway should be considered if an R0 resection is achievable [14]. As in other tracheal pathology, preoperative workup includes CT imaging of the neck and chest, bronchoscopy to identify tracheal involvement, and careful operative planning. In patients with limited tumour burden, 5-year survival is enhanced with complete resection. These patients demonstrate a more durable response to surgery with fewer symptoms and prolonged relief of airway obstruction [14]. The majority of these cases can be resected without significant morbidity or mortality and without negative effects on voice or swallowing function [15]. In cases with upper trachea or laryngeal involvement, referral to a tracheal center is advised to avoid a palliative pathway in patients who might be resectable.
Consideration for limited or local resection is not recommended due to the significant risk of locoregional recurrence [14]. In our experience, concurrent resection of an invasive thyroid malignancy into the trachea yields better results than reoperation and tracheal resection for local recurrence following limited resection [16]. In cases with extensive regional disease, limited resection may be considered but only as a palliative option. On rare occasions, non-curative radical procedures may be necessary for significant or recurrent symptoms in a patient with otherwise acceptable operative risk [15].
Other secondary tracheal neoplasms, such as upper lobe lung cancers with invasion into the mediastinum, may be candidates for resection. However, these tumours often invade the central airways and require carinal pneumonectomy, a topic outside the scope of this chapter. Additional pathologic lesions, such as squamous cell carcinoma of the larynx and esophagus, or metastasis from remote sites, such as the breast, colon, kidney, and uterus, may be resected using the above principles in patients who meet appropriate criteria. The utility of adjuvant radiation therapy following resection, even for patients who undergo palliative procedures, provides a durable outcome that may improve quality of life [17].
18.3.1 Surgical Technique
Invasive thyroid carcinoma into the trachea is approached via a transcervical technique similar to that previously described. In brief, circumferential tracheal mobilization is performed in the immediate area of invasion without tumour disruption. If there is evidence of invasion at the level of the cricoid cartilage, it may need to be resected. Preservation of the RLNs that enter on the posterolateral side of the cartilage is mandatory. Reconstruction is performed as previously described with Vicryl stay sutures on the lateral wall of the trachea and circumferential 4-0 Vicryl sutures placed in an interrupted fashion.
We routinely perform circumferential resection of the trachea and do not recommend limited resection of the sidewall as a definitive approach. “Keyhole” resections, when patched with autologous non-tracheal tissue, are at risk for poor healing and excessive granulation buildup [14]. To achieve appropriate and negative margins, create a tracheal reconstruction in a durable manner without tension, and to ensure preserved speech and swallowing function, circumferential resection and repair is preferred.
18.3.2 Results
We retrospectively evaluated 82 patients that presented to our institution with invasive thyroid carcinoma of the larynx and/or trachea. 69 of those patients underwent tracheal resection and en bloc thyroidectomy with an operative mortality of 1.2 % (1/82 patients). The incidence of anastomotic dehiscence was 4.3 % (3/69 patients). Mean survival following reconstruction was 9.4 years, with a 10-year overall survival of 40 %. Complete resection was associated with improved overall survival (17.9 vs. 13.1 years) and disease-free survival (14.6 vs. 5.1 years) compared with thyroidectomy, limited resection, and delayed resection of an airway recurrence. Positive prognostic factors included concurrent airway resection with thyroidectomy, an R0 resection, and well-differentiated tumours. Negative prognostic factors included airway symptoms, metastasis at presentation, disease recurrence, and the need for a salvage operation [11, 14, 18, 19].
18.4 Post-intubation/Subglottic Stenosis
The incidence of post-intubation stenosis has decreased significantly over the past two decades, due to reduced utilization of high-pressure ET tube cuffs causing cicatricial mucosal necrosis [20]. However, even high-volume low-pressure ET tube cuffs increase a patient’s risk for post-intubation stenosis and upper airway obstruction. Many of these cases can be avoided by the early use of tracheostomy [20, 21].
Patients with subglottic stenosis present with progressive shortness of breath, dyspnea on exertion, wheezing that can progress to stridor, or symptoms of intermittent obstruction [22]. Symptoms follow removal of the ET tube, although the majority of patients present months or even years after their hospitalization [23]. Because the ET tube passes through the mouth, concern for concurrent laryngeal injury should be considered before surgically managing subglottic stenosis. In these cases, we recommend assessment by an otolaryngologist to document proper glottic function.
Post-intubation lesions occur at four levels: (1) in the cuff site, (2) the stoma site (if tracheostomy is present), (3) the segment between the stoma and the cuff, and (4) the point where the tip of the endotracheal tube makes contact with the mucosa [20]. Stenosis at the cuff is the most common post-intubation injury and is due to circumferential pressure causing mucosal sloughing. In time healing is associated with cicatricial stenosis [20]. Stenosis at the tracheal stoma occurs after accumulation of granulation tissue, resulting in airway deformity. Obstructive symptoms become more prominent with removal of the tracheostomy [20]. Additional areas of stenosis include a posterior depressed flap of trachea above the stoma or an anterolateral stenosis. These may be caused by a large stomal defect created at the time of placement, erosion by the tracheostomy appliance into the anterior airway, or weight from ventilation tubing causing pressure necrosis and erosion. If there is significant upward pressure of the tracheostomy on the cricoid, loss of cartilage integrity can lead to concurrent subglottic and upper tracheal stenoses [24]. Tracheal malacia can also develop, most often between the cuff and stoma, and is due to loss of tracheal wall integrity associated with pooling of respiratory secretions and bacterial overgrowth [20]. Finally, the tip of the endotracheal tube may cause granuloma formation by direct contact with the tracheal mucosa, resulting in cicatricial healing and stenosis causing symptoms [25].
In patients with persistent symptoms of upper airway obstruction and a history of intubation or in cases of suspected idiopathic subglottic stenosis, workup includes CXR, CT scan with 3D reconstruction, bronchoscopy with assessment of the laryngeal and tracheal airway, and evaluation of glottic function. Imaging should be reference against intraoperative bronchoscopy (Figs. 18.2, 18.3, 18.4, and 18.5).
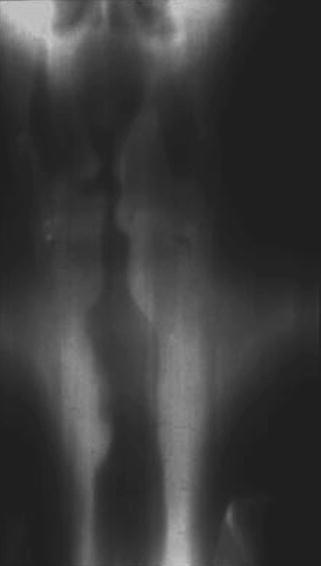
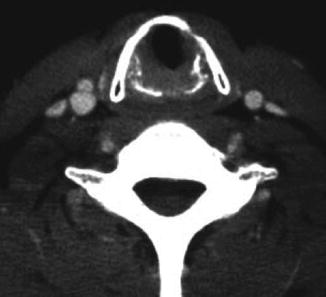
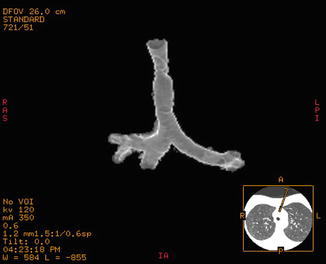
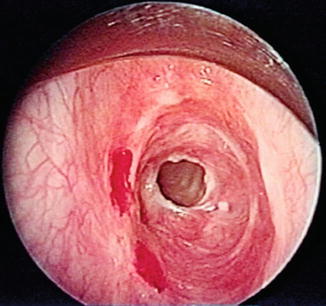

Fig. 18.2
Anteroposterior view demonstrating tracheal narrowing in a patient with post-intubation stenosis

Fig. 18.3
Axial CT imaging demonstrating tracheal narrowing in a patient with a subglottic stenosis

Fig. 18.4
CT 3D rendering of a post-intubation stenosis of the mid-trachea

Fig. 18.5
Rigid bronchoscopic view of a patient with subglottic stenosis
Acute airway obstruction should be managed by expeditious oral intubation with the tip of the endotracheal tube positioned above the stenosis; no attempt should be made to intubate through a significant narrowing. In the case of a tight subglottic stenosis, a laryngeal mask airway (LMA) may be more practical. After securing the airway and ensuring hemodynamic stability, the patient is brought to the operating room for rigid bronchoscopy and dilation. No attempt should be made to surgically address a subglottic stenosis in the acute setting. Patients who are hemodynamically stable can be extubated and admitted to a monitored setting for observation, supplemental oxygen, and chest physical therapy. We discourage the use of laser ablation in an obstructive presentation of subglottic stenosis; formation of granulation tissue or scar can be significant after laser therapy and may complicate future definitive reconstructive procedures.
Patients who may benefit from surgical intervention should be evaluated in the operating room and with favorable anatomy considered for laryngotracheal resection and reconstruction (technique is described below). For those patients with significant comorbidities or inoperable pathology, palliative placement of a tracheostomy or Silastic T tube beyond a tight stenosis should be considered.
Contraindications to resection are few but include significant comorbidities that preclude tolerance of general anaesthesia, ventilator dependence, or disease that will lead to tracheostomy placement in the future regardless of surgical success (neurologic disorders). Patients that require long segmental resection precluding a tension-free anastomosis are also not operative candidates. Deferral of definitive therapy is recommended in patients on high-dose steroids due to risk of anastomotic breakdown.
18.4.1 Surgical Technique
We have demonstrated that single-stage resection and reconstruction of a laryngotracheal stenosis can be performed successfully with maintenance of laryngeal function [26]. Stenotic airway lesions that involve the larynx require a modified technique for repair compared to mid-tracheal lesions [27]. Approach to this resection increases risk for RLN injury at its entry on posterior aspect of the cricoid cartilage.
Lesions that abut the cricoid without proximal spread can undergo simple tracheal resection and reconstruction as previously described. On the contrary, disease that abuts the vocal cords requires a staged reconstructive plan with the assistance of an otolaryngologist and may require tissue or bone grafts. Disease that does not extend proximal to the cricoid cartilage, with either isolated involvement of the anterior cartilaginous surface or circumferential disease, can be considered for laryngotracheal resection and will be discussed in further detail.
Following induction of anaesthesia, rigid bronchoscopy and dilation may be necessary to allow passage of a 5 or 6 ET tube. Dilation should be limited to prevent laryngeal edema. The technique for distal tracheal transection, cross-field ventilation, and mobilization of the posterior membrane off the esophagus and surrounding structures has been previously described. In the case of an anterior subglottic stenosis, dissection includes circumferential mobilization of the trachea limited to the area of resection. Pretracheal dissection to the level of the carina is not necessary, especially for a short-segment stenosis.
If the entire length of the anterior cricoid cartilage is involved, it must be resected. This includes dissection of the cricothyroid muscle off either side of the cartilage and division of the lateral cricoid laminae. The proximal resection margin includes the anterior arch of the cricoid cartilage, including the cricothyroid membrane if necessary, to the level of the lateral laminae (Fig. 18.6). The distal tracheal margin should be fashioned to accommodate the beveled proximal cartilage, beveling the distal ring backward from the anterior midline to posterior cartilage (Fig. 18.7). Tailoring should only be performed through a single ring and does not extend into the posterior membrane. In some cases, the distal resection should be performed after laryngeal division; a larger posterior membranous flap might be necessary to resurface the posterior cricoid plate and will be discussed below. Reconstruction is performed as described, including placement of 2-0 Vicryl traction sutures in the distal airway and larynx and interrupted 4-0 Vicryl sutures for reconstruction. Suture placement at the laryngeal margin must pass through the mucosa to ensure approximation with the tracheal mucosa. Some amount of laryngeal cartilage should also be captured to allow for strong reapproximation. Traction sutures placed in the larynx must have enough cartilaginous purchase to avoid pulling through during reapproximation; at the same time, they should be superficial enough not enter the airway lumen.