Fig. 11.1
The HeartMate XVE (Thoratec Inc., Pleasanton, CA) first-generation pulsatile LVAD and HeartMate II axial flow LVAD. (Courtesy of Thoratec Corp., used with permission.)
Several LVADs with third-generation design have entered clinical trials. These include the HVAD (HeartWare Corp., Framingham, MA), the Duraheart (Terumo Heart Inc., Ann Arbor, MI), the Levacor VAD (World Heart Corp., Salt Lake City, Utah), and the VentrAssist device (Thoratec Corp., Pleasanton, CA) Figs. 11.2. In a recent review, third-generation devices were characterized by a noncontact bearing design, in contrast to second-generation axial flow devices which have a contact bearing configuration [54]. Noncontact design is achieved by suspension of the rotor using either magnetic or hydrodynamic levitation, or a combination of both. The theoretical advantage is the elimination of contacting parts and friction wear, with even greater potential for improved long-term durability. These devices also offer the advantages of small size and small percutaneous drivelines. The HeartWare HVAD is small enough in size to be implanted completely within the pericardial space, eliminating the need for a preperitoneal pocket. Third-generation design offers the promise of additional improvements in long-term device reliability, an important factor in achieving greater acceptance of LVADs for destination therapy.
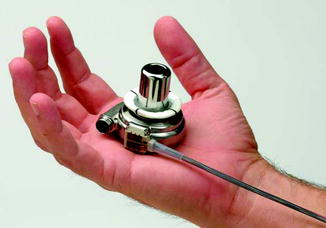

Fig. 11.2
The HeartWare HVAD (HeartWare Corp., Framingham, MA). (Courtesy of HeartWare Inc., used with permission.)
Current Outcomes
The REMATCH trial established definitively the survival and quality-of-life benefit with mechanical circulatory support for the treatment of end-stage heart failure [7]. The limitations of first-generation device therapy, however, were highlighted by the increased incidence of adverse events in LVAD patients. In this trial, 129 patients suffering from New York Heart Association (NYHA) class IV heart failure who were not candidates for cardiac transplantation were randomized to either optimal medical management or placement of a left VAD (HeartMate VE LVAD, Thoratec Inc., Pleasanton, CA). Inclusion criteria for the trial included left ventricular ejection fraction of <25 %, need for continuous intravenous inotropic therapy, and a peak oxygen consumption of <12 ml/kg/min. The primary end point was death from any cause, with several secondary end points such as the incidence of adverse events, days of hospitalization, quality of life, and functional status. Device therapy led to a 48 % reduction in the risk of death from any cause. Survival rates for the device group and medically treated group at 1 year were 52 % and 25 %, respectively, and 23 % and 8 % at 2 years. In addition, assessments of quality of life and functional status were significantly better in the device group. The probability of device failure was 35 % at 2 years, with ten patients in the trial requiring device exchange.
During the course of the REMATCH trial, as clinical experience was gained and device enhancements made, outcomes improved. Park and colleagues analyzed the clinical results of patients undergoing device placement as stratified by era of the trial [10]. Patients receiving devices in the second half of the trial had 1- and 2-year survivals of 59 % and 38 %, as compared to 44 % and 21 % for patients undergoing LVAD placement in the first half of the trial. The overall rate of adverse events was also significantly lower in the late cohort of LVAD patients. This included decreased rates of sepsis, pump housing infection, renal failure, and perioperative bleeding.
Since the REMATCH trial, continued progress has been towards improving clinical results and decreasing the morbidity and mortality associated with device therapy. Lietz and coworkers reported the results of 280 patients undergoing LVAD placement for destination therapy following conclusion of the REMATCH trial [31]. The complete data of 222 of these patients was used to derive a preoperative risk score for 90-day in-hospital mortality. Using the risk score, patients were then stratified into four categories (low, medium, high, and very high risk). Overall survival in the group of post-REMATCH patients was 56 % and 31 % at 1 and 2 years, respectively. When stratified by risk categories, 1-year survival rates were 81.2 %, 62.4 %, 27.8 %, and 10.7 % for low-, medium-, high-, and very high-risk groups, respectively (Fig. 11.3). Survival to hospital discharge was 87.5 % in the low-risk group as compared to only 10.7 % in the very high-risk cohort. This analysis highlights the importance of patient selection as one of the critical determinants of successful outcomes with VAD therapy.
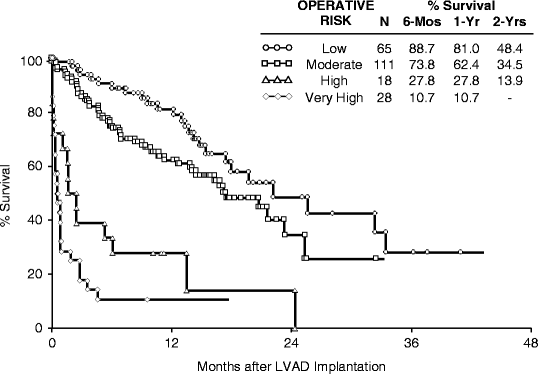

Fig. 11.3
LVAD survival stratified by DT risk score (adapted from Lietz K, Long JW, Kfoury AG, Slaughter MS, Silver MA, Milano CA, Rogers JG, Naka Y, Mancini D, Miller LW. Outcomes of left ventricular assist device implantation as destination therapy in the post-REMATCH era: implications for patient selection. Circulation. 2007; 116: 497–505.)
Long and colleagues reported their single-center experience with destination therapy [14]. When compared to the REMATCH trial, there were significantly improved survival rates of 77 % seen at 1- and 2-year follow-up (Fig. 11.4). In this group of patients, perioperative mortality was decreased to 8.1 %, compared to 31 % for patients in the REMATCH trial. There was also a significant reduction in the incidence of adverse events. Authors attributed the continuing improvement in destination therapy in the modern era to improvements in patient management, patient selection, and device design enhancements.
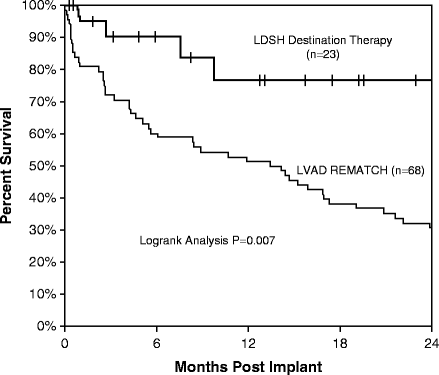

Fig. 11.4
Destination therapy survival at LDS Hospital, Salt Lake City, UT versus REMATCH trial (adapted from Long JW, Healy AH, Rasmusson BY, Cowley CG, Nelson KE, Kfoury AG, Clayson SE, Reid BB, Moore SA, Blank DU, Renlund DG. Improving outcomes with long-term “destination” therapy using left ventricular assist devices. J Thorac Cardiovasc Surg. 2008;135(6):1353–1361.)
The development of second-generation axial flow pumps represents a significant advance in device therapy. Single-center experiences have shown reduced rates of morbidity and mortality with axial flow devices [55]. While the prediction, avoidance, and treatment of right ventricular dysfunction following LVAD placement remain a challenge, Patel and colleagues demonstrated a reduced need for RVAD implantation following LVAD placement with axial flow devices compared to pulsatile first-generation devices [56].
One of the largest clinical trials evaluating the use of mechanical circulatory support for destination therapy has recently completed enrollment. Patients in this trial were randomized to receive either the continuous-flow HeartMate II axial flow device or the pulsatile-flow HeartMate XVE LVAD in a 2:1 ratio. Preliminary results of the trial indicate significant advantages of the second-generation HeartMate II when compared to the HeartMate XVE, leading to the termination of randomization prior to completion of the trial. Several third-generation devices, as previously discussed, have entered clinical trials in the USA.
Conclusions
VAD therapy has emerged as an important treatment option for patients suffering from end-stage heart failure. Since the results of the REMATCH trial, acceptance of LVADs as destination therapy has been slow. As experience with patient selection and management has grown and as device design has evolved, clinical outcomes with regard to survival and freedom from adverse events continue to improve. Efforts toward the development of smaller devices, transcutaneous energy sources, and minimally invasive implantation techniques are aimed at reducing complications associated with current device therapy. This will likely lead to an increase in the acceptance in the use of LVADs earlier in the progression of heart failure, potentially leading to further improvements in clinical outcomes. In addition, more widespread dissemination of the technology will improve access of therapy to the many patients who could benefit from LVAD support, likely leading to an increase in the use of devices for long-term destination therapy in patients not eligible for cardiac transplantation.
References
1.
Hunt SA, Abraham WT, Chin MH, et al. ACC/AHA 2005 Guideline Update for the Diagnosis and Management of Chronic Heart Failure in the Adult-Summary Article. Circulation. 2005;112(12):1825–52.CrossRef
3.
4.
5.
6.
The International Society for Heart & Lung Transplantation. ISHLT Online. http://www.ishlt.org. Accessed 9 Jan 2013.
7.
8.
9.
Pae WE, Connell JM, Adelowo A, Clinical Utility Baseline Study (CUBS) Group, et al. Does total implantability reduce infection with the use of a left ventricular assist device? The LionHeart experience in Europe. J Heart Lung Transplant. 2007;26:219–29.PubMedCrossRef
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


