A subset of adult patients with an open atrial septal defect (ASD) have pulmonary arterial hypertension (PAH). We sought to identify predictors of response to PAH-specific medical therapy in this group. Invasive hemodynamic and clinical parameters from 12 patients with an open ASD and PAH (pulmonary vascular resistance [PVR], 8.8 ± 1.2 Wood units; mean pulmonary artery pressure, 55 ± 6 mm Hg; Qp:Qs ratio, 1.1 ± 0.1; and 6-minute walk test distance of 1,046 ± 116 feet) were analyzed. Responders (n = 5) underwent successful ASD closure at 1.3 ± 0.3 years after initiation of medical therapy and were characterized by >30% reduction in PVR (7.2 ± 1.5 to 4.6 ± 0.9 Wood units) versus <20% in nonresponders (n = 7; 9.9 ± 1.7 to 8.2 ± 1.5 Wood units, p <0.03), increased 6-minute walk test distance (1,087 ± 174 vs 1,405 ± 109 feet, p = 0.05), and higher Qp:Qs ratio after therapy (1.9 ± 0.2 vs 1.1 ± 0.2, p <0.02). Body mass index was a significant clinical predictor of response (23.3 ± 1.9 vs 30.0 ± 2.1 kg/m 2 , p <0.05) and the change in arterial saturation with exercise correlated inversely with change in PVR (r = −0.739, p <0.01). In conclusion, medical therapy led to a significant improvement in hemodynamic and clinical parameters in a subset of patients with an open ASD and PAH, who were able to safely undergo delayed ASD closure.
In the last decade, there has been a striking shift in treatment options for pulmonary arterial hypertension (PAH) including prostacyclins, endothelin receptor antagonists, and phosphodiesterase (type 5) inhibitors. This has led to decreased morbidity and increased survival of not only patients with idiopathic-PAH but also those with PAH and underlying congenital heart disease (CHD). These treatments may lead to improved hemodynamics in patients with an atrial septal defect (ASD) and PAH (ASD-PAH) and, thereby, facilitate closure candidacy. Several case reports have shown that adults with bidirectional and right-to-left shunts who were treated medically with PAH-specific therapy, subsequently underwent successful ASD closure. None have examined potential predictors of favorable response to medical therapy and subsequent ASD closure. Our objectives were to summarize the first descriptive case series of invasively confirmed patients with ASD-PAH at a tertiary care center, determine response to PAH-specific medical therapy, identify potential clinical predictors of this response, and report closure candidacy or outcome in this group.
Methods
We conducted a retrospective review of consecutive adults aged >18 years at our institution that had an established diagnosis of ASD-PAH (1998 to 2012). Informed consent was obtained as part of the Washington University Center for Adults with Congenital Heart Disease database and the institutional review board approved this study. A database query for “atrial septal defect” and “pulmonary hypertension” and review of patient records from the institutional Pulmonary Vascular Center and Center for Adults with Congenital Heart Disease was undertaken. Inclusion criteria were documented open ASD, mean pulmonary artery (PA) pressure ≥25 mm Hg, pulmonary vascular resistance (PVR) ≥3 Wood units, pulmonary capillary wedge pressure ≤15 mm Hg, absence of complex CHD, and no other cause for PAH. All hemodynamic measurements were obtained invasively with the use of a PA catheter at the time of right-sided cardiac catheterization. One patient with mixed PAH and pulmonary venous hypertension had normal left ventricular function, no greater than grade 1 diastolic dysfunction, and pulmonary capillary wedge pressure ≤18 mm Hg in the setting of high PVR or mean PA pressure and was included.
Decisions regarding medical therapy were made on an individual basis taking into account all clinical data. At our institution all patients are treated according to recommendations published by the American College of Cardiology Foundation and American Heart Association expert consensus regarding longitudinal evaluation of PAH including clinic visits every 3 to 6 months with functional class assessment and 6-minute walk test (6MWT), periodic echocardiography or cardiac magnetic resonance imaging to assess the right ventricle, and right-sided cardiac catheterization with change in clinical status or to assist with decision making regarding treatment options. We use the following general criteria for closure: PVR <6 Wood units, ratio of PVR to systemic vascular resistance <0.3, and net left-to-right shunt with Qp:Qs ratio >1.5. It is also our practice that patients who do not meet these criteria for closure or demonstrate exercise-induced hypoxemia (saturation <90%) be treated with medical therapy aimed at lowering PVR and undergo subsequent hemodynamic reevaluation in the following 6 to 12 months, in accordance with current recommendations. Closure is typically recommended to prevent development or progression of PAH, reduce the risk of atrial enlargement and subsequent arrhythmia, and to prevent paradoxical emboli. Patients who satisfied the aforementioned criteria after a period of medical therapy were offered closure of the ASD by the most appropriate method for their individual defect.
The following data were collected: age, gender, type of ASD, other CHD, type or date of closure if applicable, and medical therapy. Objective data (echocardiogram, invasive hemodynamics, 6MWT, and modified World Health Organization functional classification) were collected when available at the following intervals: before medical therapy (open ASD), latest follow-up after initiation of medical therapy but before closure, and after closure.
Descriptive data were generated and characteristics of long-term treatment responders (met institutional closure criteria after medical therapy) and nonresponders (did not meet institutional closure criteria after medical therapy) were evaluated with 1-way analysis of variance for continuous variables and with Fisher’s exact tests for categorical and ordinal variables. Within-group change was evaluated with paired t tests. Data were examined for normality according to the Shapiro-Wilks criteria, and non–normally distributed data sets were log-transformed before statistical analyses were performed. All significance tests were evaluated with type I error rate of 5% (α = 0.05). All data are presented as means with SEM. The data were analyzed using SAS v9.3 (SAS Institute Inc., Cary, North Carolina).
Results
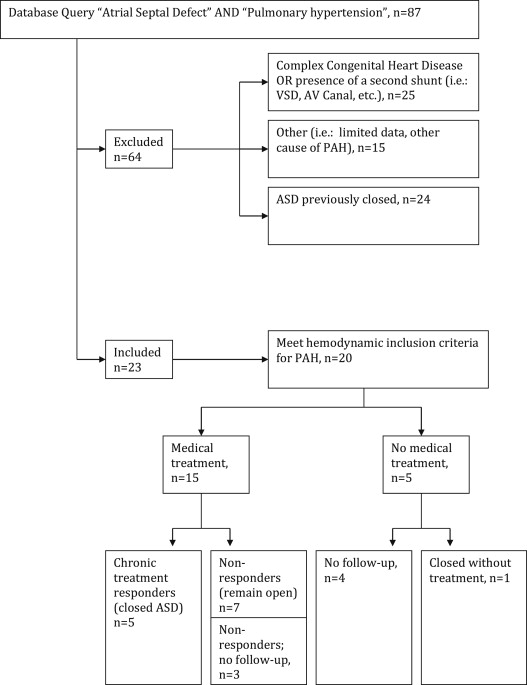
Eighty-seven patients were initially identified, 20 had an open ASD (13 secundum, 6 sinus venosus, and 1 primum) and met inclusion criteria. Medical treatment was given to 15 patients, and of these, 5 underwent successful closure after PAH-specific medical therapy, whereas 7 remained on medical therapy without ASD closure (3 were lost to follow-up). Of the 20 identified patients, 5 received no medical therapy. In that group, 1 patient underwent ASD closure and the remaining (n = 4) were lost to follow-up ( Figure 1 ).
Most patients who received medical therapy were on a single medication (n = 8). The remaining patients received a combination therapy with either 2 (n = 6) or 3 (n = 1) PAH-specific medications. The 3 classes of pulmonary vasodilators were prescribed with equal frequency (phosphodiesterase [type 5] inhibitors, n = 7; endothelin receptor antagonists, n = 9; and prostacyclin, n = 7). Baseline demographics and clinical characteristics of patients with an open ASD-PAH are listed in Table 1 .
| Patient Identification | Responders (n = 5) | Nonresponders (n = 7) | p | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | Mean ± SEM | 1 | 2 | 3 | 4 | 5 | 6 | 7 | Mean ± SEM | ||
| Demographics | |||||||||||||||
| Age (yrs) | 63 | 22 | 30 | 33 | 22 | 34 ± 7 | 28 | 52 | 57 | 65 | 45 | 38 | 47 | 48 ± 5 | 0.14 |
| Women, n (%) | Women | Men | Women | Men | Women | 3 (60%) | Women | Men | Women | Men | Women | Men | Women | 4 (57%) | 0.69 |
| BMI (kg/m 2 ) | 26.3 | 27.2 | 15.9 | 25.0 | 25.2 | 23.3 ± 1.9 | 26.3 | 22.3 | 36.5 | 35.4 | 28.3 | 28.2 | 30.7 | 30.0 ± 2.1 | 0.04 |
| Clinical history or data | |||||||||||||||
| ASD type | S | S | S | S | S | S | S | SV | SV | S | SV | S | |||
| Other CHD | None | None | None | PAPVR | None | None | None | PAPVR | PAPVR | None | PAPVR | None | |||
| Age of closure | 31 | 24 | 33 | 35 | 24 | N/A | N/A | N/A | N/A | N/A | N/A | N/A | |||
| Type of closure | P | S, F | P, F | S, F | P | N/A | N/A | N/A | N/A | N/A | N/A | N/A | |||
| Initial therapy | ERA | P | ERA | PDE5, ERA | PDE5, ERA | P | PDE5 | P | PDE5, ERA | PDE5, ERA | P, PDE5, ERA | PDE5, ERA | |||
| NYHA class, median (range) | 3 | 3 | 2 | 2 | 3 | 3 (2–3) | 4 | 2 | 3 | 2 | 2 | 2 | 2 | 2 (2–4) | 0.22 |
| Atrial tachycardia, n (%) | No | No | No | No | No | 0 (0%) | No | No | Yes | No | No | Yes | No | 2 (29%) | 0.32 |
| Smoker >10 yrs, n (%) | Yes | No | Yes | No | Yes | 3 (60%) | No | Yes | No | Yes | No | No | Yes | 3 (43%) | 0.50 |
| Respiratory disease, n (%) | No | No | No | No | Yes | 1 (20%) | No | Yes | No | Yes | Yes | Yes | No | 4 (67%) | 0.18 |
| BNP (pg/ml), median (range) | 14 | 89 | 3,938 | 78 | N/A | 84 (14–3,938) | N/A | 149 | 171 | 36 | 535 | 34 | N/A | 149 (34–535) | 0.40 |
| Hemoglobin (g/dl) | 10.9 | 15.8 | 17.9 | 14.5 | 11.9 | 14.2 ± 1.3 | 16.9 | 17.6 | 16.1 | 15.0 | 11.2 | 15.5 | 14.3 | 15.2 ± 0.8 | 0.49 |
| Resting O 2 saturation (%) | 96 | 88 | 100 | 99 | 100 | 97 ± 2 | 95 | 99 | 98 | 99 | 96 | 96 | 100 | 98 ± 1 | 0.65 |
| 6MWT distance (feet) | 1,200 | 625 | 750 | 1,550 | 1,310 | 1,087 ± 174 | 700 | 1,575 | 350 | 1,440 | 750 | 1,200 | 1,100 | 1,116 ± 165 | 0.78 |
| Echocardiographic data | |||||||||||||||
| LA volume (ml/m 2 ) | 27 | 13 | 29 | 18 | 17 | 21 ± 3 | 17 | 15 | 28 | 24 | 13 | 16 | 20 | 19 ± 2 | 0.62 |
| RA volume (ml/m 2 ) | 44 | 49 | 58 | 41 | 36 | 46 ± 4 | 83 | 55 | 56 | 54 | 23 | 49 | 36 | 51 ± 7 | 0.57 |
| Severe TR, n (%) | No | No | No | No | No | 0 (0%) | Yes | No | Yes | No | Yes | No | No | 3 (43%) | 0.16 |
| TAPSE (cm) | 1.7 | N/A | N/A | 2.4 | 2.5 | 2.2 ± 0.3 | N/A | 1.2 | N/A | 1.9 | N/A | 2.4 | N/A | 1.8 ± 0.4 | 0.43 |
| RVMPI | 0.72 | 1.03 | 1.44 | 0.33 | 0.38 | 0.80 ± 0.2 | 1.03 | 1.60 | 1.12 | 0.84 | 1.20 | 0.49 | 0.50 | 1.0 ± 0.20 | 0.47 |
| PA diameter (cm) | 2.5 | 3.0 | 3.1 | 3.6 | 2.7 | 2.9 ± 0.2 | 4.0 | 3.4 | 4.8 | 3.0 | 2.8 | 4.4 | 3.0 | 3.6 ± 0.3 | 0.12 |
| dP/dt (mm Hg/s) | 440 | 500 | 600 | 360 | 1,090 | 598 ± 129 | 480 | 570 | 480 | 545 | 480 | 916 | 830 | 614 ± 69 | 0.91 |
| Invasive hemodynamics | |||||||||||||||
| RA pressure (mm Hg) | 6 | 7 | 12 | 1 | 8 | 7 ± 2 | 6 | 5 | 10 | 9 | 6 | 9 | 3 | 7 ± 1 | 0.98 |
| mPA pressure (mm Hg) | 34 | 92 | 57 | 48 | 57 | 58 ± 10 | 45 | 53 | 100 | 37 | 52 | 55 | 35 | 54 ± 8 | 0.78 |
| PVR (Wood units) | 3.8 | 4.6 | 12.0 | 6.3 | 9.1 | 7.2 ± 1.5 | 7.1 | 18.9 | 11.5 | 7.5 | 7.9 | 10.3 | 6.1 | 9.9 ± 1.7 | 0.27 |
| Qp:Qs | 1.4 | 0.5 | 1.8 | 1.9 | 1.0 | 1.3 ± 0.2 | 0.78 | 0.8 | 1.0 | 1.3 | 1.3 | 0.9 | 0.8 | 1.0 ± 0.1 | 0.19 |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


