Renal dysfunction is related to long-term mortality and myocardial infarction after coronary artery bypass grafting (CABG). We aimed to investigate the association between preoperative renal dysfunction and early risk of stroke, myocardial infarction, or heart failure after CABG. From the Swedish Web-system for Enhancement and Development of Evidence-based care in Heart disease Evaluated According to Recommended Therapies registry, we included all 36,284 patients who underwent primary isolated CABG from 2000 to 2008 in Sweden. The Swedish National Inpatient Registry was used to obtain the primary end point, which was rehospitalization for stroke, myocardial infarction, or heart failure ≤90 days after CABG. Logistic regression models were used to estimate the risk for the primary outcome and the secondary outcome of death from any cause, while adjusting for confounders. During 90 days of follow-up, there were 2,462 cardiovascular events and 617 deaths. In total, 17% of patients developed acute kidney injury postoperatively. Odds ratios with 95% confidence intervals for cardiovascular events after adjustment for age, gender, atrial fibrillation, left ventricular ejection fraction, diabetes mellitus, peripheral vascular disease, and history of myocardial infarction, heart failure, or stroke was 1.24 (1.06 to 1.45) in patients with an estimated glomerular filtration rate of 15 to 45 ml/min/1.73 m 2 but became nonsignificant after acute kidney injury was introduced into the statistical model. The risk of death was significantly increased in patients with estimated glomerular filtration rate of 15 to 45 ml/min/1.73 m 2 (odds ratio 1.76, 95% confidence interval 1.38 to 2.25) even after adjustment for all confounders. Renal dysfunction was associated with all-cause mortality but not with cardiovascular events during the first 3 postoperative months after primary isolated CABG.
Chronic kidney disease (CKD) is a growing public health issue with >10% of the general population affected. Because the average life span is increasing and diabetes is also becoming more prevalent, the prevalence of CKD will even further increase in the future. Subjects with CKD have an increased risk of cardiovascular events and premature death.
Renal dysfunction is common in patients who undergo coronary artery bypass grafting (CABG) and affects 20% to 40% of subjects in different study populations. Renal dysfunction is also strongly related to early and long-term mortalities and increases the risk of myocardial infarction and stroke in the longer term after CABG. However, less is known regarding the association between renal dysfunction and stroke, myocardial infarction, or heart failure in the first postoperative months after CABG.
Studies on early outcome are often limited to in-hospital or 30-day follow-ups. Even if the patients survive to discharge or 30 days, there may be an increased risk of adverse outcome in the following postoperative months. If this is the case, it may be reasonable to follow the patient more closely for the first few months after surgery. This study aimed to assess the impact of renal dysfunction on the risk of stroke, myocardial infarction, heart failure, or death within the first 3 months after CABG.
Methods
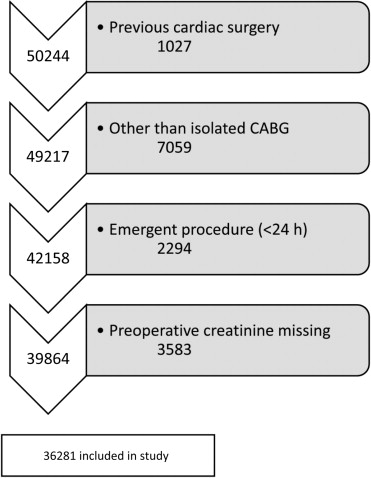
All patients who underwent a first isolated CABG from 2000 to 2008 in Sweden were included from the Swedish Web-system for Enhancement and Development of Evidence-based care in Heart disease Evaluated According to Recommended Therapies (SWEDEHEART) registry. In this registry, all patients undergoing cardiac surgery in Sweden are registered since 1992. The SWEDEHEART registry has been described in detail previously, and the agreement between data in the registry and medical records is almost complete. Patients with previous cardiac surgery, surgical procedures being performed in addition to CABG, patients who underwent emergent surgery, patients who were dialysis dependent or had an estimated glomerular filtration rate (eGFR) of <15 ml/min/1.73 m 2 , and patients with missing information on preoperative creatinine values were excluded ( Figure 1 ). The study complied with the Declaration of Helsinki and was approved by the Regional Ethical Review Board in Stockholm.
Serum creatinine values were usually analyzed the day before surgery. To estimate glomerular filtration rates (eGFRs), we used the simplified 4-variable Modification of Diet in Renal Disease study formula: eGFR = 186 × (serum creatinine) −1.154 × (age) −0.203 × (0.742 if female). Renal function was divided into the following categories: >60 ml/min/1.73 m 2 , 45 to 60 ml/min/1.73 m 2 , and 15 to 45 ml/min/1.73 m 2 . Acute kidney injury was defined according to the Acute Kidney Injury Network criteria as >0.3 mg/dl (26 μmol/L) or 50% to 100% increase (stage 1), 100% to 200% increase (stage 2), or >200% increase (stage 3) in the postoperative serum creatinine value compared with the preoperative value. In the SWEDEHEART registry, the highest postoperative serum creatinine value during the entire hospitalization is registered.
The Swedish National Inpatient Registry registers all patients who are hospitalized in Sweden and was started in 1964. This registry has covered the whole country since 1987. The discharge diagnosis in this registry is coded by the physician who discharges the patient from hospital. The diagnosis of stroke, myocardial infarction, or heart failure as the principal cause of hospitalization in the Swedish National Inpatient Registry has been validated previously and found to have a high sensitivity and positive predictive value. Stroke, myocardial infarction, or heart failure was defined as a hospitalization for any of these diagnoses during follow-up. Information about hospitalization before surgery with a discharge diagnosis of myocardial infarction, stroke, heart failure, diabetes mellitus, atrial fibrillation, and chronic obstructive pulmonary disease was also ascertained from the Swedish National Inpatient Registry, using data from 1964 and onwards. The date of death was determined from the Swedish Total Population Register (Statistics Sweden). Patients were followed from the day of surgery until they were discharged with a principal diagnosis of stroke, myocardial infarction, or heart failure, until their death, or 90 days postoperatively, whichever came first.
Patient characteristics are presented as means with 1 SD and proportions in relation to different categories of renal function. Univariate and multivariate logistic regression models were used to calculate odds ratios (ORs) for cardiovascular events, death, or a combination of cardiovascular events or death in the following categories of renal function: eGFR of 45 to 60 ml/min/1.73 m 2 and 45 to 15 ml/min/1.73 m 2 . The reference category was an eGFR of >60 ml/min/1.73 m 2 .
The final model included the following variables: age (continuous), gender (male = 0, female = 1), atrial fibrillation (yes or no), left ventricular ejection fraction (normal, moderate, or poor), diabetes mellitus (yes or no), peripheral vascular disease (yes or no), previous stroke (yes or no), previous myocardial infarction (yes or no), previous heart failure (yes or no), and perioperative acute kidney injury categorized according to the Acute Kidney Injury Network criteria. Data were missing on left ventricular function in 13% of the patients, peripheral vascular disease in 13%, postoperative creatinine values in 18%, and diabetes in 28%. We used multiple imputation by chained equations to impute missing values for preoperative left ventricular ejection fraction (normal, moderate, or poor), diabetes mellitus (yes or no), peripheral vascular disease (yes or no), and acute kidney injury (yes or no). One hundred data sets were imputed and estimates from these data sets were combined. In addition, we performed a complete case analysis in which only observations without missing values for model covariates were included (n = 20,823).
Stata, version 12.1 (StataCorp LP, College Station, Texas), was used for all analyses.
Results
A total of 36,281 patients with a mean age of 67 years were included; of whom, 22% were women. Twenty percent of patients had an eGFR of <60 ml/min/1.73 m 2 . There were 15% of patients who had moderately reduced eGFR (45 to 60 ml/min/1.73 m 2 ) and 5% who had severely reduced eGFR (15 to 45 ml/min/1.73 m 2 ). Patients with reduced eGFR were older, were more often women, and had more co-morbidities ( Table 1 ).
| Variable | All Patients | eGFR (ml/min/1.73 m 2 ) | p ∗ | ||
|---|---|---|---|---|---|
| >60 | 45–60 | 15–45 | |||
| No. of patients | 36,284 | 28,847 | 5,532 | 1,902 | |
| Percent of study population | 100 | 80 | 15 | 5 | |
| Age (yrs) | 66.9 ± 9.3 | 65.7 ± 9.2 | 71.4 ± 7.9 | 72.1 ± 8.5 | <0.001 |
| Women | 22% | 18% | 34% | 43% | <0.001 |
| eGFR (ml/min/1.73 m 2 ) | 76 ± 21 | 83 ± 17 | 54 ± 4 | 37 ± 7 | |
| Serum creatinine (μmol/L) | 92 ± 26 | 84 ± 14 | 113 ± 15 | 160 ± 43 | |
| Serum creatinine (mg/dl) | 1.0 ± 0.3 | 0.9 ± 0.2 | 1.3 ± 0.2 | 1.8 ± 0.5 | |
| Diabetes mellitus | 23% | 21% | 26% | 37% | <0.001 |
| Atrial fibrillation | 2% | 2% | 3% | 3% | <0.001 |
| EuroSCORE | 3.8 ± 2.5 | 3.5 ± 2.3 | 5.0 ± 2.3 | 5.9 ± 2.6 | <0.001 |
| Hypertension | 56% | 54% | 64% | 74% | <0.001 |
| Hyperlipidemia | 58% | 57% | 59% | 67% | <0.001 |
| Peripheral vascular disease | 8% | 7% | 11% | 19% | <0.001 |
| Current smoking | 19% | 20% | 14% | 16% | <0.001 |
| Chronic obstructive pulmonary disease | 6% | 6% | 7% | 9% | <0.001 |
| Previous myocardial infarction | 45% | 43% | 51% | 58% | <0.001 |
| Previous heart failure | 4% | 3% | 7% | 12% | <0.001 |
| Previous stroke | 5% | 4% | 6% | 11% | <0.001 |
| Left ventricular ejection fraction | |||||
| >50% | 71% | 73% | 65% | 55% | <0.001 |
| 30%–50% | 26% | 24% | 30% | 38% | <0.001 |
| <30% | 4% | 3% | 5% | 7% | <0.001 |
| Year of surgery | |||||
| 2000–2004 | 60% | 57% | 69% | 66% | <0.001 |
| 2005–2008 | 40% | 43% | 31% | 34% | <0.001 |
Reoperation for bleeding or deep wound infection was more common among patients with a reduced eGFR. In total, 17% of the study population developed acute kidney injury postoperatively. In patients with an eGFR of >60 ml/min/1.73 m 2 , 14% developed acute kidney injury compared with 34% and 42% in patients with moderately and severely reduced eGFR, respectively. Operative and postoperative characteristics are listed in Table 2 .
| Variable | All Patients | eGFR (ml/min/1.73 m 2 ) | p ∗ | ||
|---|---|---|---|---|---|
| >60 | 45–60 | 15–45 | |||
| No. of patients | 36,284 | 28,847 | 5,532 | 1,902 | |
| Percent of study population | 100 | 80 | 15 | 5 | |
| Internal thoracic artery use | 94% | 94% | 94% | 92% | <0.001 |
| No. of grafted coronary arteries, mean ± SD | 3.3 ± 1.0 | 3.3 ± 1.0 | 3.4 ± 1.1 | 3.4 ± 1.1 | 0.03 |
| CABG without cardiopulmonary bypass | 6% | 6% | 7% | 9% | <0.001 |
| Reoperation for bleeding | 6.1% | 5.7% | 7.5% | 8.7% | <0.001 |
| Reoperation for sternal wound complications | 3.7% | 3.3% | 5.2% | 5.5% | <0.001 |
| Acute kidney injury † | |||||
| No kidney injury | 83% | 86% | 76% | 58% | <0.001 |
| Stage 1 | 15% | 13% | 21% | 38% | <0.001 |
| Stage 2 | 1.4% | 1.1% | 2.2% | 3.8% | <0.001 |
| Stage 3 | 0.4% | 0.4% | 0.3% | 0.3% | 0.685 |
∗ Chi-square test or 1-way analysis of variance.
† Acute kidney injury classified according to the Acute Kidney Injury Network classification. Stage 1: 0.3 to 0.5 mg/dl or 50% to 100% increase, stage 2: 100% to 200% increase, and stage 3: >200% increase in postoperative compared with preoperative creatinine values.
There were 2,462 cardiovascular events during 90 days of follow-up. A total of 617 patients (1.5%) died within 3 months of surgery. The unadjusted risk for cardiovascular events increased from 51% in those with moderately reduced eGFR by more than twofold in patients with severely reduced eGFR ( Table 3 ). After adjustment for all confounders except acute kidney injury, the association was still significant for severely reduced eGFR but became nonsignificant when acute kidney injury was introduced into the statistical model ( Table 3 ).



