The long-term outcome of athletes with frequent ventricular premature complexes (VPCs) and apparently normal heart has not been fully clarified. To evaluate the clinical and prognostic significance of VPCs and the influence of continuing sports activity during follow-up, we studied 120 healthy athletes (96 men; median age 16 years) in whom frequent VPCs (>100 VPCs/24 hours) were discovered by chance during preparticipation screening. All athletes were followed up for a median of 84 months. During follow-up, 96 underwent serial 24-hour Holter recording and 62 underwent serial echocardiography. The median number of VPCs/24 hours on basal Holter was 3,760. During follow-up, 81 athletes continued sports activity, whereas 39 did not. No athlete died or developed overt heart disease. The median number of VPCs/24 hours decreased in both athletes who continued sports activity and those who did not (from 3,805 to 1,124, p <0.0001 and from 5,787 to 1,298, p <0.0001, respectively). During follow-up, left ventricular ejection fraction slightly decreased to <55% in 9 of 62 athletes who, in respect to the remaining 53, had more VPCs/24 hours both in the basal state (12,000 vs 3,880) and during follow-up (10,702 vs 1,368), and a longer follow-up (95 vs 36 months). In conclusion, (1) frequent VPCs in athletes without heart disease have a long-term benign prognostic significance, (2) sporting activity does not modify this benign outcome, (3) during follow-up, the burden of VPCs decreases whether or not subjects continue sports activity, and (4) in 14.5% of athletes, ejection fraction slightly decreases over time.
Frequent ventricular premature complexes (VPCs) may be discovered by chance in otherwise healthy athletes during preparticipation screening. As the long-term outcome of these subjects has not been fully clarified, in this study, we evaluated their clinical prognosis and the influence of continuing sports activity on the complexity of arrhythmias during a follow-up of several years.
Methods
We analyzed 205 competitive athletes, <35 years old, consecutively referred to the arrhythmologic center of our division of cardiology from several sports medicine centers in Italy, after the discovery of ventricular premature beats during screening for eligibility for sport. Cases were collected from 1979 to 2008.
In accordance with the Italian screening program, all athletes had undergone medical examination, standard 12-lead electrocardiography (ECG), and submaximal exercise testing. When enrolled in our center, all athletes underwent echocardiography, 24-hour Holter monitoring, and maximal exercise testing. Further instrumental evaluations were decided on a clinical basis.
Forty-five subjects were excluded from the study because they presented ≥1 of the following: family history of juvenile (<40 years) sudden death or hereditary cardiomyopathies, syncope, hypertension, or any kind of heart disease such as right ventricular cardiomyopathy, mitral valve prolapse with significant valvular insufficiency, hypertrophic cardiomyopathy, or dilated cardiomyopathy. Furthermore, subjects were excluded if they had sustained (>30 seconds) ventricular tachycardia (SVT), rapid (shortest RR <300 ms) nonsustained ventricular tachycardia (NSVT), or iterative right or left ventricular outflow tract tachycardia. The criteria used for the diagnosis of arrhythmogenic right ventricular cardiomyopathy (ARVC), hypertrophic cardiomyopathy, mitral valve prolapse, ventricular outflow tract tachycardia, and so forth are those commonly recommended. All these athletes were excluded from sport activity and entrusted to their respective clinical cardiologists.
Of the remaining 160 athletes, 20 were excluded because they had <100 VPCs/24 hours on Holter monitoring. Another 20 were excluded because they had been enrolled <1 year before the date of the last follow-up examination. Thus, 120 athletes (96 men; median age 16 years, interquartile range [IQR] 13 to 26) took part in the study ( Figure 1 ). Most athletes played soccer (42%); the remainder played volleyball (14%), basketball (11%), or other sports (33%). All athletes played competitively.
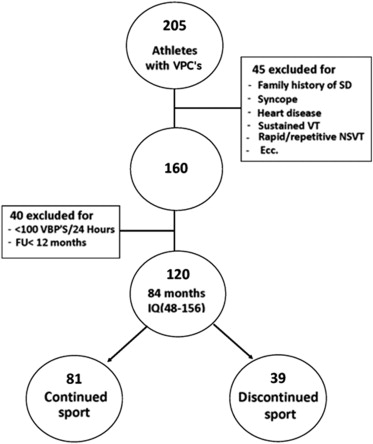
All had a normal electrocardiogram according to Corrado et al. Specifically, early repolarization patterns, negative T wave limited to V 1 , and high voltages in V 5 and V 6 in endurance athletes were regarded as normal findings. All participants also had normal echocardiogram and maximal effort test results.
The morphology of VPCs was defined as left bundle branch block (LBBB) and right bundle branch block (RBBB)-like. In the presence of QRS duration of <0.12 second with LBBB or RBBB morphology (with or without anterior or posterior fascicular block), a fascicular morphology was defined, suggesting an origin within the conduction system ( Figure 2 ).
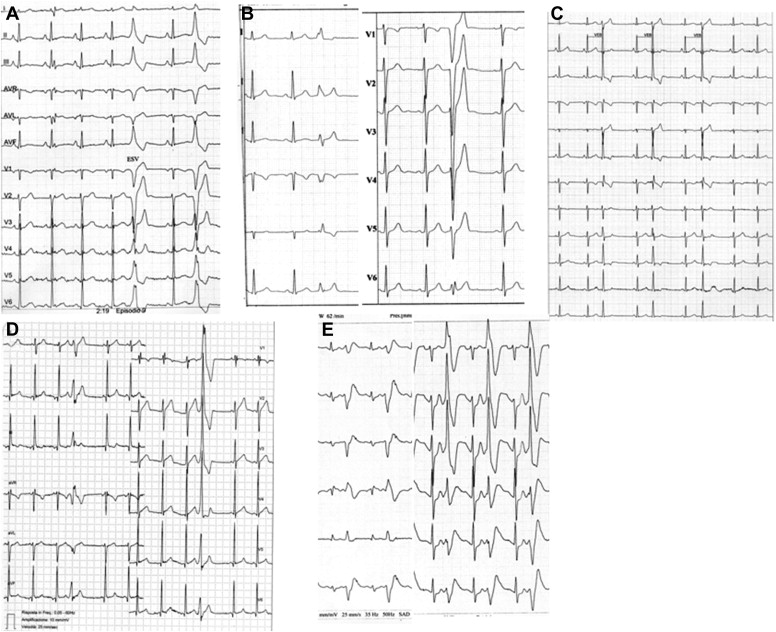
In subjects with >1 morphologic pattern, the prevalent morphology was considered. After evaluation, we suggested to give eligibility to sport to all athletes, on the basis of the absence of heart disease and malignant arrhythmias. However, the final decision was made by the individual sports medicine doctors who had sent us the athletes. All participants were scheduled in our center and invited to undergo periodic (if possible, annual) examinations. All athletes were called for a final clinical-instrumental examination from 2008 to 2011. All 120 subjects were available for the final clinical follow-up examination. During follow-up, 96 athletes underwent serial (≥2) 24-hour Holter recordings. In addition, 62 athletes underwent serial (≥2) echocardiography.
Statistical analyses were made using the SYSTAT 13 (2009, Systat Software, Chicago, Illinois) packages. For continuous variables, comparisons among groups were made by Student t test or analysis of variance. Pearson chi-square test was used for categorical variables. Log transformations were used to correct for positive-skewed distributions, as appropriate. Cut-off values were obtained using receiver operating characteristic curve analysis. Data are presented as median and interquartiles for continuous measures and as proportion for categorical variables. All p values are 2-tailed, and statistical significance was established as p <0.05.
Results
Despite normal electrocardiogram, echocardiogram, and effort testing, generally after the enrollment during follow-up, 18 athletes (15%) underwent further instrumental examinations: cardiac magnetic resonance imaging (14) right ventricular CARTO (Biosense Webster, Yokneam, Israel) mapping (5), right ventriculography (2), and coronary angiography (2). In all cases, these additional examinations proved normal.
Basal characteristics of Holter monitoring are listed in Table 1 . In brief, the median number of VPCs/24 hours on basal 24-hour Holter monitoring was >3,700. 35.8% had couplets and 14% had NSVT. The median RR intervals of couplets and NSVT were 400 and 450 ms, respectively, whereas the shortest RR intervals were 300 and 320 ms, respectively. The median number of beats of NSVT was 4 (IQR 2 to 6).
| Characteristic | Value |
|---|---|
| No. of athletes | 120 |
| Men/Women | 96/24 |
| Age | |
| Median | 16 |
| IQR | 13–26 |
| VPCs/24 h | |
| Median | 3,760 |
| IQR | 1,340–8,141 |
| Morphology of VPCs | |
| LBBB (LBBB + RAD) | 83 (50) |
| RBBB | 7 |
| Fascicular | 24 |
| Not available | 6 |
| Couplets | |
| No. of athletes (%) | 43 (35.8) |
| Median/24 h in 43 athletes | 14 |
| IQR | 4–60 |
| NSVT | |
| No. of athletes (%) | 17 (14) |
| Median/24 h in 17 athletes | 1 |
| IQR | 1–2 |
The prevalent morphology of VPCs on 12-lead electrocardiogram was precisely identified in 114 cases ( Table 1 ). The prevalent morphology (72.8%) was LBBB, followed by fascicular morphology (21%) and RBBB (6.1%). The 2 prevalent morphologies (i.e., LBBB and fascicular) did not correlate with gender (p = 0.40 and p = 0.47, respectively), basal number of VPCs (p = 0.27 and p = 0.69, respectively), couplets (p = 0.29 and p = 0.09, respectively), or NSVT/24 hours (p = 0.20 and p = 0.23, respectively). In contrast, subjects with fascicular morphology were younger than the remaining subjects (median 13, IQR 11 to 17 vs median 17, IQR 13 to 26, p = 0.01), whereas subjects with LBBB morphology were older than the remaining subjects (median 18, IQR 13 to 27 vs median 13, IQR 11 to 18, p = 0.01).
During effort testing at increasing loads, VPCs disappeared in 72% of cases and reappeared during recovery; in 24% of cases, VPCs remained stable during the test, whereas in 4%, they increased.
All athletes were called for a final clinical examination.
The median duration of clinical follow-up was 84 months (IQR 48 to 156), that is, 7 years. In 49 athletes (40.8%), follow-up continued for ≥10 years. Ninety-six athletes underwent at least 2 Holter recordings during follow-up (median 3, IQR 2 to 4). The remaining 24 subjects, after basal evaluation, declined to undergo further instrumental examinations. Among the former, the last Holter recording was performed a median of 36 months (IQR 19 to 71) after enrollment.
Comparison between basal and last Holter recordings is listed in Table 2 . In brief, the median number of VPCs/24 hours significantly decreased, and in 29 athletes (30%), VPCs either totally disappeared (12) or became sporadic (<100 ventricular premature beats/24 hours). Subjects in whom VPCs disappeared or became sporadic during follow-up did not differ significantly in terms of gender, age, morphology, or the number of VPCs on basal Holter from those who continued to have >100 VPCs/24 hours ( Table 3 ). The only difference was the longer follow-up in the former and, obviously, the median number of VPCs/24 hours during follow-up.
| Basal | Follow-Up, Median 36 mo (IQR 19–71) | p | |
|---|---|---|---|
| No. of VPCs/24 h | |||
| Median | 4,198 | 1,240 | <0.0001 |
| IQR | 1,730–9,243 | 15–7,255 | |
| <100 VPCs/24 h | 0 | 29 | |
| Couplets | |||
| No. of athletes | 35 | 35 | 0.15 |
| Median/24 h | 15 | 4 | 0.63 |
| IQR | 4–60 | 1–20 | |
| NSVT | |||
| No. of athletes | 16 | 11 | 0.31 |
| Median/24 h | 1 | 1 | 0.32 |
| IQR | 1–2 | 1–1 |
| Characteristic | VPCs/24 h During Follow-Up | p | |
|---|---|---|---|
| <100, n = 29 | >100, n = 67 | ||
| Women (%) | 28 | 18 | 0.28 |
| Age | |||
| Median | 14 | 16 | 0.55 |
| IQR | 12–23 | 13–24 | |
| Follow-up duration (mo) | |||
| Median | 50 | 36 | 0.02 |
| IQR | 33–87 | 14–60 | |
| Basal VPCs/24 h | |||
| Median | 3,300 | 4,357 | 0.33 |
| IQR | 1,549–7,939 | 1,760–10,232 | |
| FU VPCs/24 h | |||
| Median | 2 | 3,699 | <0.0001 |
| IQR | 0–11 | 1,055–9,750 | |
| Morphology (%) | |||
| LBBB vs all others | 34 | 31 | 0.76 |
| Fascicular vs all others | 24 | 24 | 0.97 |
Couplets were present in the basal state and at the end of follow-up in a similar number of athletes, but the median number of couplets decreased. The number of athletes with sporadic NSVT decreased at the end of follow-up. On considering all Holter recordings, no subject developed SVT.
Of the 96 athletes who underwent serial Holter monitoring during follow-up, 58 were still practicing sport at the time of the last Holter recording, whereas 38 had discontinued sport activity at least 4 months earlier (median 12, IQR 5 to 28).
The trends in arrhythmias in these 2 groups of subjects, from the baseline to the end of follow-up, are listed in Table 4 . From the baseline to the last Holter recording during follow-up, the median number of VPCs/24 hours significantly decreased in both athletes who continued sporting activity and those who did not ( Figure 3 ). In addition, the percentage of subjects in whom VPCs disappeared or became sporadic (<100 VPCs/24 hours) during follow-up was similar in both groups (31% vs 29%).
| Sport Yes (58) | Sport No (38) | |||||
|---|---|---|---|---|---|---|
| Basal | End FU | p | Basal | End FU | p | |
| No. of VPCs/24 h | ||||||
| Median | 3,805 ∗ | 1,124 † | <0.0001 | 5,787 ∗ | 1,298 † | <0.0001 |
| IQR | 1,800–7,893 | 11–7,060 | 1,556–10,300 | 18–7,452 | ||
| No. of athletes with <100 VPCs/24 h | 0 | 18 ‡ (31%) | 0 | 11 ‡ (29%) | ||
| Couplets | ||||||
| No. of athletes | 17 § | 23 ¶ | 0.18 | 18 § | 12 ¶ | 0.35 |
| Median/24 h | 17 | 4 | 0.52 | 13 | 3 | 0.43 |
| IQR | 5–101 | 1–20 | 4–31 | 1–20 | ||
| NSVT | ||||||
| No. of athletes | 10 ‖ | 12 ∗∗ | 0.09 | 6 ‖ | 4 ∗∗ | 0.36 |
| Median/24 h | 1 | 1 | 0.17 | 1 | 1 | 0.32 |
| IQR | 1–3 | 1–1 | 1–1 | 1–1 | ||
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


