Umbilical Cord Blood Gases to Assess Fetal Condition at Birth
The assessment of the fetal condition at birth had traditionally been restricted to the Apgar score. It is interesting to note that this score, proposed originally by its namesake, Virginia Apgar, an anesthesiologist, was developed primarily to assess the need for newborn resuscitation (1). However, as investigators became interested in the influence of the process of labor and delivery on the long-term development of affected newborns, investigators began to assess the utility of the Apgar score in predicting adverse neurologic development. It became apparent that low Apgar scores, except in the extreme, had little predictive ability for such adverse outcomes. For example, an Apgar score of 0 to 3 at 5 minutes only increases the risk of cerebral palsy (CP) by 1%, whereas with an Apgar score of 0 to 3 even at 10 minutes, 90% of children did not develop CP (2,3). This should not be surprising as the primary complications in term babies, which are likely to adversely affect long-term development of the brain, are hypoxia and acidosis (perinatal asphyxia). But a low Apgar score is only a reflection of neurologic depression, of which acidosis may certainly be one cause, but is far from the only cause. Other causes of newborn depression include drugs or anesthetic agents; anomalies affecting the fetal brain, lungs, airway, or heart; airway obstruction; sepsis; pulmonary hypoplasia; and even the resuscitation process itself, as with intubation and suctioning for meconium. Thus, there should be and is an alternative for assessing the fetus at birth and that is the evaluation of umbilical cord blood gases. The assessment of the fetus for the presence, absence, and degree of acidosis at birth not only is potentially useful for the prediction of longterm sequelae but also has other important implications. For the depressed baby who is not acidotic, it guides the pediatrician to look for other causes of depression. If the baby is acidotic, it will assist the pediatrician in tailoring the resuscitative efforts. For the obstetrician, the assessment of umbilical blood gases will help, at least in retrospect, with the interpretation of the fetal heart rate (FHR) tracing, as will be discussed later in this chapter. In the case of a baby who later develops any neurologic dysfunction, the cord blood gases can be used to refute or confirm the possibility of perinatal asphyxia as a contributing cause, and this has implications for the treating neurologist as well as in the courtroom if the child’s damage results in a malpractice suit. Thus, the evaluation of umbilical cord blood gases has become an important and common method of evaluating the depressed baby at birth.
PHYSIOLOGY OF FETAL ACID-BASE BALANCE
pH is a logarithmic representation of the quantity of hydrogen ions in fluid. Specifically, pH is the negative logarithm of the hydrogen ion concentration. So a pH of 7.0 is equal to a molar hydrogen ion concentration of 10-7 or, expressed numerically, .0000001 M H+. In humans, it is critical that hydrogen ions are kept in a close balance both by the rate of production and by a process of either conversion or buffering with substances in blood and tissue, which prevents excess hydrogen ions from doing harm. The major buffers in blood are hemoglobin and bicarbonate. Hemoglobin essentially absorbs hydrogen ions and thus has a finite capacity to simply absorb acid and carry it to the kidneys where it can be excreted. In the fetus, the kidney’s ability to excrete acid is relatively immature, and therefore, its role in acid buffering is minimal once the hemoglobin capacity has been saturated. The other major method of buffering excess H+ is by the conversion of bicarbonate to carbonic acid (H2CO3) by the formula:
Under normal circumstances, metabolism of sugars produces carbon dioxide (CO2) and water, and CO2 produced by the fetus is eliminated across the placenta. Under conditions of hypoxia, as with the adult, the fetus converts energy with a process known as anaerobic metabolism, which involves the conversion of sugars and fatty acids into pyruvic and lactic
acids resulting in excess H+ ion production and a lowering of the pH. To prevent harm from these hydrogen ions to cells, tissues, and organs, these excess H+ ions must be buffered by the mechanisms described above. As the pH drops during such anaerobic metabolism, the shift in the equation results in reduced bicarbonate and loss of this buffering capacity. Thus, the H+ content increases (a drop in pH), and when this falls out of the normally very tight range of safe pH, an acidosis results. This type of acidosis resulting from an increased production of hydrogen ions by anaerobic metabolism (or by the direct production of other organic acids as in diabetic ketoacidosis) is called metabolic acidosis. Both hydrogen ions and the bicarbonate that buffers them are highly charged ions and move very slowly across the placenta. Thus, with a metabolic acidosis, the placenta’s ability to clear hydrogen ions from the fetus or replenish bicarbonate from the mother is a relatively slow process, and a significant metabolic acidosis, when it is finally resolved, will take many hours for the placenta alone to reverse it.
acids resulting in excess H+ ion production and a lowering of the pH. To prevent harm from these hydrogen ions to cells, tissues, and organs, these excess H+ ions must be buffered by the mechanisms described above. As the pH drops during such anaerobic metabolism, the shift in the equation results in reduced bicarbonate and loss of this buffering capacity. Thus, the H+ content increases (a drop in pH), and when this falls out of the normally very tight range of safe pH, an acidosis results. This type of acidosis resulting from an increased production of hydrogen ions by anaerobic metabolism (or by the direct production of other organic acids as in diabetic ketoacidosis) is called metabolic acidosis. Both hydrogen ions and the bicarbonate that buffers them are highly charged ions and move very slowly across the placenta. Thus, with a metabolic acidosis, the placenta’s ability to clear hydrogen ions from the fetus or replenish bicarbonate from the mother is a relatively slow process, and a significant metabolic acidosis, when it is finally resolved, will take many hours for the placenta alone to reverse it.
The other type of acidosis seen in the fetus is known as respiratory acidosis. This results from an inability to clear from the blood stream the normally produced CO2. In the fetus, the most common reason for this is compression or other reduction in flow in the umbilical cord vessels; although if the mother develops a respiratory acidosis, the fetus will accumulate more CO2 and the gradient is changed as well. The fetus, as with the adult, produces CO2 as a result of oxidative metabolism of sugars and fatty acids under normal well-oxygenated circumstances. The CO2 produced by the fetus cannot be eliminated, as in the adult, through the lungs, so it is eliminated through the placenta into the maternal circulation and out of the maternal respiratory tract. As long as there is normal umbilical blood flow, placental exchange, and maternal respiration, the CO2 diffuses very rapidly across the placenta. Normal umbilical arterial pCO2 (reflecting that blood exiting the fetus to the placenta) has a value of about 50 mm Hg, whereas the venous pCO2 exiting the placenta has a value of about 40 mm Hg. This relatively large difference demonstrates how rapidly CO2 is cleared through the placenta. A respiratory acidosis reflecting retained CO2 in the fetus, most commonly due to umbilical cord compression or other obstruction to umbilical blood flow, will clear very rapidly when the compression is released as the CO2 in the umbilical artery is rapidly diffused across the placenta, the equilibrium of the equation is shifted away from the production of hydrogen ions, and the pH moves toward normal.
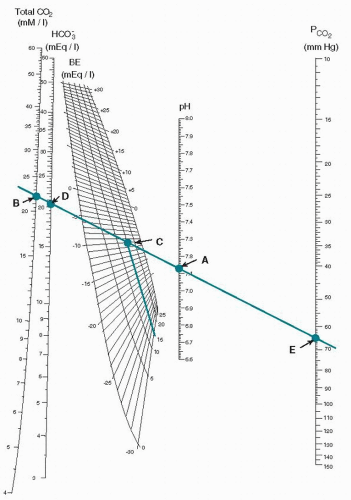
When the pH is low, it is important to distinguish between a respiratory acidosis and a metabolic acidosis; the bicarbonate value that can be measured is not an accurate reflection in and of itself. The concept of base excess or base deficit is used for this purpose. The pH value and the measured pCO2 are plotted on a curve known as the Siggaard-Andersen normogram (Fig. 7.1). Now that measurement of the pH and pCO2 is automated, this calculation is most commonly done on the same instrument that has a computer built in for this purpose. As seen in Figure 7.1, a line is drawn through the pCO2 (point E) and the pH (point A), and the base excess is read from the plot at point C. The plot is variable based on the fetal hemoglobin concentration, and the base excess is read from the plot. An elevated base excess for an umbilical cord is >—9, and thus a low pH with an elevated base excess would be indicative of a metabolic acidosis and a low pH with a normal base excess would be a respiratory acidosis. In the latter case, the pCO2 would be elevated, and in the former, the pCO2 would be normal. A mixed respiratory and metabolic acidosis will have both an elevated base excess and an elevated pCO2. The clinical picture of the baby at birth with a respiratory acidosis, depending on the severity, will be a low 1-minute Apgar score and, following resuscitation, a relatively normal 5-minute Apgar score, as the efforts of resuscitation will clear the elevated CO2 rapidly and allow the pH to return to normal. A metabolic acidosis, when relatively severe, will be reflected in both low 1- and 5-minute Apgar scores. This is because with resuscitation, only after oxygen (O2) reaches the cells and tissues is the anaerobic metabolism reversed and buffers are restored that the pH becomes normal, and this process takes considerably longer. It is important to review the terminology as this is often confusing, and care must be used to express the status of fetal blood gases and their results as accurately as possible (Table 7.1).
Technique for Obtaining Umbilical Cord Blood Gases
To collect umbilical cord blood for analysis, a doubly clamped segment of the cord is cut away from the placenta immediately after delivery of the baby. Delay in clamping the cord segment for obtaining cord blood gases can significantly alter the pH and pCO2. The blood samples can be obtained with disposable plastic syringes that have been flushed with heparin (1,000 U per cm3). It is important to carefully expel all the heparin from the syringes, as heparin can alter the pH. Samples are obtained from both the umbilical artery and vein whenever possible. Umbilical arterial blood is often more difficult to obtain. If this proves to be the case, blood can be obtained from an artery on the surface of the placenta. The arteries are identified as they override the vein on the placental surface. Samples should be carefully labeled as umbilical artery or vein and with the time they are obtained. The samples are stable either in the clamped segment of the cord or in the syringe for up to 60 minutes and do not have to be transported to the laboratory on ice.
Normal Cord Blood Gas Values
The blood from the umbilical artery reflects information on the blood returning to the placenta from the fetus, so it is most informative about the state of the fetus at the time
of delivery. Blood from the venous side will be useful in confirming the accuracy of the values obtained from the artery and, as will be discussed, if an acidosis does exist confirming whether the acidosis is metabolic or respiratory. The values that are most important are the pH and the pCO2. The pO2 is only useful in confirming that the specimen obtained was actually from the umbilical cord and not from an umbilical venous catheter obtained from the newborn sometime later, as fetal pO2 is substantially lower than newborn values. The pO2 in and of itself is not informative because the level fluctuates so much minute to minute, especially in the final minutes before birth, and even very vigorous and subsequently normal fetuses can have very low pO2 values.
of delivery. Blood from the venous side will be useful in confirming the accuracy of the values obtained from the artery and, as will be discussed, if an acidosis does exist confirming whether the acidosis is metabolic or respiratory. The values that are most important are the pH and the pCO2. The pO2 is only useful in confirming that the specimen obtained was actually from the umbilical cord and not from an umbilical venous catheter obtained from the newborn sometime later, as fetal pO2 is substantially lower than newborn values. The pO2 in and of itself is not informative because the level fluctuates so much minute to minute, especially in the final minutes before birth, and even very vigorous and subsequently normal fetuses can have very low pO2 values.
TABLE 7.1 Terminology used to describe blood gases in the fetus | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Normal values for the umbilical cord artery and vein are shown in Table 7.2 (4,5 and 6). Values for term and preterm babies are similar (7).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree