Tricuspid Valve Disease
Tricuspid valve (TV) disease is often secondary to, or in association with, mitral or aortic valve disease or left ventricular (LV) disease and receives less attention than the primary left-sided disease. It is frequently labeled “the forgotten valve” because surgical correction is often ignored. Appropriate treatment of the TV disease, even when secondary to left heart diseases, may improve long-term functional outcome.1
The TV is the most apically (or caudally) placed valve with largest orifice among the four valves. The TV apparatus includes leaflets or cusps, chordae and papillary muscles, and tricuspid annulus in addition to the right atrium (RA) and right ventricle (RV).
The tricuspid annulus is oval in shape and becomes more circular when dilated. The annulus has a complex nonplanar shape with the posteroseptal portion being the lowest and the anteroseptal being the highest.2 It tends to become more planar with moderate or severe “functional” tricuspid regurgitation (TR). The annular orifice area is approximately 20% larger than the mitral annulus area, with a major diameter of 3.0 to 3.5 cm in adults. The larger orifice provides for the inflow to occur at lower velocities and lower pressure decreases. Both early and late diastolic velocities are lower than the mitral inflow. The annulus expands in diastole and constricts in midsystole with a nearly 30% reduction in annular area.3
In general, the TV has three distinct leaflets described as septal, anterior, and posterior. The septal and the anterior leaflets are larger. The posterior leaflet is smaller and appears to be of lesser functional significance because it may be imbricated without impairment of valve function. The septal leaflet is in immediate proximity to the membranous ventricular septum, and its extension provides a basis for spontaneous closure of the perimembranous ventricular septal defect. The larger anterior leaflet is attached to the anterolateral margin of the annulus and is often voluminous and sail-like in Ebstein anomaly.
There are three sets of smaller papillary muscles; each set is composed of up to three muscles. The chordae tendineae arising from each set are inserted into two adjacent leaflets. Thus, the anterior set chordae insert into half of the septal and half of the anterior leaflets. The medial and posterior sets are similarly related to adjacent valve leaflets.
The diastolic valve opening with expansion of the annular orifice provides for unimpeded inflow. Although systolic narrowing of the orifice is intended to result in effective valve closure; some degree of valvular regurgitation with color Doppler imaging is quite common. Nearly 50% to 60% of young adults exhibit mild TR. A smaller proportion of normal adults, up to 15%, have moderate TR. The significance of mild or moderate TR should be evaluated on the basis of clinical findings.
TV disease or dysfunction is generally classified as primary (ie, intrinsic) and secondary valve pathology.4,5 The latter is secondary to left heart disease and resulting RV hypertension, dilatation, and dysfunction. It is also described as functional TR.
Congenital
- Cleft valve in association with atrioventricular (AV) canal defect
- Ebstein anomaly
- Congenital tricuspid stenosis
- Tricuspid atresia
- Cleft valve in association with atrioventricular (AV) canal defect
Rheumatic valve disease, generally in association with rheumatic mitral disease
Infective endocarditis
Carcinoid heart disease
Toxic (eg, Phen-Fen valvulopathy or methysergide valvulopathy)
Tumors (eg, myxoma)
Iatrogenic—pacemaker lead trauma
Trauma—blunt or penetrating injuries
Degenerative—TV prolapse
RV dilatation
RV hypertension (ie, pulmonary hypertension)
RV dysfunction with cardiomyopathy, myocarditis, or chronic RV hypertension
Segmental dysfunction secondary to ischemia or infarction of the RV, endomyocardial fibrosis, arrhythmogenic RV dysplasia.
The common causes of RV hypertension, dilatation, and failure are from left heart disease in form of advanced mitral, aortic, and LV myocardial disorders. Thus, TR is most commonly secondary to conditions affecting the left heart and is caused by annular dilatation and leaflet tethering with reduction in surface of leaflet coaptation. In more advanced cases, the leaflets fail to coapt, with a resulting wide-open TR.
The functional derangement may be in form of (1) pure or predominant tricuspid stenosis, (2) pure or predominant TR, or (3) mixed.
Generally, the symptoms of left heart disease predominate in those with secondary TV disease. The symptoms specific to advanced TV disease are related to (1) decreased cardiac output (eg, fatigue) or (2) RA hypertension (eg, liver congestion resulting in right upper quadrant discomfort or gut congestion with symptoms of dyspepsia, indigestion, or fluid retention with leg edema and ascites). It may be emphasized that significant TV disease may not be associated with any symptoms until a late stage of the disease involving progressive RV dysfunction.
These include signs related to TV disease and those secondary to chronic venous congestion, that is, leg edema, ascites.
Tricuspid stenosis results in characteristic changes in the jugular venous pulse in form of a slow V to Y descent and prominent “a” waves. The liver is enlarged with a firm edge and is pulsatile in presystole. Auscultation reveals a low- to medium-pitched diastolic rumble with inspiratory accentuation. This is usually localized to the lower sternal border (see Chap. 14).6
TR results in the jugular venous pulse exhibiting prominent C-V wave or systolic wave. There is often a parasternal lift from RV enlargement.7,8 The liver shows systolic pulsations, is enlarged, and is often tender. The cardiac auscultation reveals a soft early or holosystolic murmur, which is augmented with inspiratory effort (Carvallo sign). A systolic honk may be present with TV prolapse.9 Substantial TR may exist without the classic auscultatory findings.
There are no specific markers of TV disease, although the following clues may be present: (1) RV hypertrophy and “strain” with right axis duration and (2) RA enlargement with prominent P waves.
Cardiomegaly associated with prominent right-heart borders may be noted. There are no specific findings to suggest a diagnosis of TV disease.
Two-dimensional echocardiography with spectral and color flow Doppler evaluation provides the most accurate and comprehensive laboratory test in the evaluation of TV disease (Fig. 79–1).10 The TV morphology helps differentiate primary from secondary TR.11 The right heart chamber enlargement is best visualized in apical four-chamber and subcostal views, although accurate quantitation of chamber size and ejection fraction are problematic. An indirect measure of RV ejection fraction is based on systolic displacement of the tricuspid annulus using M-mode recording.12 Tissue Doppler imaging of the annulus provides similar correlation between annular systolic velocity and RV function.13 Recent development of real-time three-dimensional echocardiography provides additional information on morphology and function of the TV.
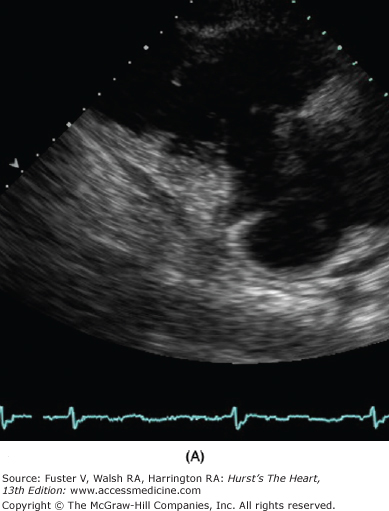
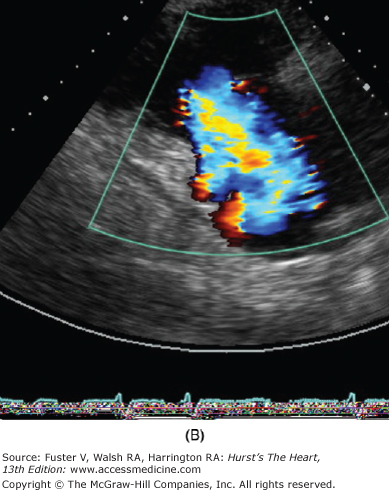
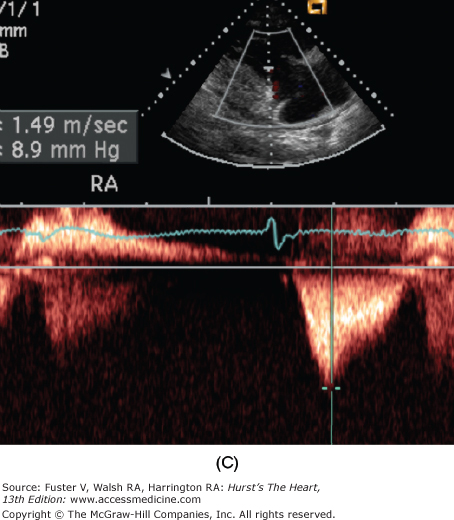
Figure 79–1.
Severe secondary tricuspid regurgitation caused by extreme tethering of the tricuspid valve without intrinsic leaflet pathology. A. The right ventricular inflow view from parasternal transducer location shows a markedly tethered valve in late systole. B. The color Doppler image shows flow accelerating and severe tricuspid regurgitation jet without turbulence. C. Continuous-wave Doppler shows early peaking systolic profile associated with high right atrial pressure, which was estimated to be 25 mm Hg. The peak tricuspid regurgitation velocity is measured at 9 mm Hg and thus indicates the right ventricular systolic pressure to be 34 mm Hg (9 + 25).
The TV morphology is best assessed using the parasternal tricuspid inflow view, apical and subcostal four-chamber views, and parasternal short-axis view. Abnormal structure and function of the valve provide insights into the likely underlying cause. The functional or secondary TR is characterized by annular dilatation, the extent of which may determine its severity.
Quantitation of valve lesion is obtained using spectral and color flow Doppler approaches. Tricuspid stenosis is detected by presence of flow acceleration on the atrial side of the valve and turbulence downstream with the RV inflow. The severity of tricuspid stenosis is based on mean and end-diastolic gradients measured using continuous-wave Doppler recordings. The normal mean gradient is less than 2 mm Hg, and the end-diastolic gradient is nearly zero. Significant stenosis of the TV may be present with a mean gradient of 3 to 5 mm Hg and an end-diastolic gradient of 1 to 3 mm Hg. The use of pressure half-time to estimate TV area and of two-dimensional echo-based planimetry of the tricuspid orifice have not been documented and are rarely, if ever, used. TR is detected using color Doppler imaging. Its severity may be semiquantitated based on the extent of the regurgitation jet penetration into the RA and inferior vena cava (IVC). Whereas the jet of the mild TR occupies up to 2 cm into the RA, the jet of moderate regurgitation extends deeper (3-5 cm) into the atrium but does not exhibit systolic reversal in hepatic or caval flow. However, with severe TR, there is consistent systolic flow reversal in the hepatic vein using the pulsed Doppler approach. A more quantitative assessment of TR may be obtained by using flow acceleration, proximal isovelocity surface area methods, and width of the vena contracta (see Chap. 18). A simpler approach is based on measuring the proximal isovelocity surface area radius. For the simpler method, the aliasing scale is adjusted at approximately one-twelfth of the peak regurgitation velocity (normally <3.0 m/s by shifting the color scale baseline to approximately 25-30 cm/s). The proximal isovelocity surface area radius at this adjusted aliasing scale of 1 to 4 mm indicates mild regurgitation, 5 to 8 mm indicates moderate regurgitation, and greater than 9 mm indicates severe regurgitation. The width of vena contracta greater than 7.0 mm is an additional indicator of severe regurgitation.
A spectral display of TR velocity is obtained using the continuous wave Doppler approach generally from RV inflow view, short-axis view, or four-chamber view. The peak velocity used to estimate RV systolic pressure is calculated as follows: RV systolic pressure = 4 × peak TR velocity + RA pressure (see Chap. 18).
The RA pressure is assumed as 7 to 10 mm Hg but may also be estimated based on the IVC size and its change with a sniff test.
The estimated RV systolic pressure correlates well with that measured at cardiac catheterization. However, the upper limit of measured peak systolic pressure using the Doppler approach is 40 mm Hg rather than the 30 mm Hg measured directly. This discrepancy is partly a result of respiratory variations and assumed RA pressure, which may vary by 3 to 5 mm Hg from the measured mean atrial pressure. It must be emphasized that the magnitude of the velocity does not indicate the severity of regurgitation but rather the height of RV systolic pressure.
Thus, a peak velocity between 3.0 and 3.9 m/s indicates moderate and in excess of 4.0 m/s severe pulmonary hypertension even if the TR severity is mild and vice versa. The shape of the velocity profile gives considerable hemodynamic information. An early peak with rapid deceleration indicates equalization of RV and RA pressures in late systole, generally from a large CV wave. A broadly rounded TR velocity profile that occupies 50% more of the R-R interval is indicative of impaired RV function.
Transthoracic echocardiography is often of diagnostic quality because the TV and the RV are closer to the anterior chest wall and several parasternal, apical, and subcostal views are used to image these structures. However, transesophageal echocardiography is indicated for better anatomic definitions of the valve lesions or precise measurement of the tricuspid annulus. The assessment of severity of tricuspid stenosis or TR is generally more accurate with transthoracic echocardiography. This is especially true in the intraoperative setting, where severity of TR may be underestimated as a result of lowered pulmonary vascular resistance from the anesthetic agents.
In the intraoperative setting, transesophageal echocardiography is especially used for measuring the tricuspid annulus diameter. This is done in the midesophageal four-chamber view and a plane perpendicular (90 degrees) to it.
Before the advent of diagnostic echocardiography, cardiac catheterization was used to confirm the presence and severity of tricuspid stenosis. It was recognized that simultaneous recordings of RA and RV diastolic pressures were needed for accurate assessment because the pressure gradients are small and there is considerable respiratory variation in the pressure wave forms. The diagnosis of TR posed a greater challenge because selective angiography into the RV would often distort the TV. The pressure wave forms in the RA showed the characteristic prominent systolic V wave with rapid descent only in the most severe cases. Diagnostic cardiac catheterization should rarely, if ever, be undertaken for the diagnosis or quantitation of TV disease alone.
The management is guided by the underlying cause and pathology of the TV. Because functional or secondary TR in association with left-heart disorders is more common, its management is emphasized here. The commonly encountered primary conditions with TV disease in adults are considered separately.
The treatment is often guided by the type and severity of the left heart disease, which determines the clinical presentation. In nearly all cases of secondary TR, the timing and approach of surgical or interventional treatment is guided by the underlying mitral, aortic, or LV disease. The medical management— consisting of digitalis, diuretics, and angiotensin-converting enzyme inhibitors—may ameliorate functional TR associated with chronic congestive heart failure.
The treatment of predominant severe mitral stenosis consists of percutaneous balloon valvuloplasty, open surgical mitral valve (MV) repair, or surgical MV replacement (see Chap. 78). In most patients, the treatment of MV stenosis alone results in a decline in pulmonary artery pressures, which continues over 6 to 12 months.14,15 The associated functional TR, depending on its severity, may impair improvement in cardiac output despite relief of the MV obstruction, especially in the early postoperative phase. TR often improves with a progressive decrease in pulmonary hypertension. However, in some patients, the severity of TR fails to regress and remains an important factor in the long-term clinical outcome. These patients often exhibit persistent right heart failure with low cardiac output.16 Most surgeons prefer to perform TV annuloplasty at the time of MV surgery. The established indication for TV annuloplasty is the presence of moderate or severe TR. This must generally be determined by transthoracic echocardiography. Intraoperative transesophageal echocardiography is less reliable for this assessment because the general anesthesia agents will cause an underestimation of TR because of their effects on pulmonary and systemic vascular resistances. There is suggestion that tricuspid annuloplasty should also be considered when the tricuspid annulus shows significant dilatation to prevent late occurrence of clinically significant TR.17 The tricuspid annulus in a normal adult has a maximum diameter of less than 3.5 cm. When this measurement exceeds 4.0 cm
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree