Fig. 8.1
An algorithm depicting the potential courses of care for patients with severe cardiogenic shock
The etiology of heart failure plays a major role in determining the ultimate course of therapy for patients with cardiogenic shock. The common etiologies of cardiogenic shock include acute myocardial infarction (AMI), decompensated chronic heart failure, postcardiotomy shock, fulminant myocarditis, peripartum cardiomyopathy, various valve disorders, and congenital defects. Patients with cardiogenic shock following AMI need to undergo prompt revascularization either by percutaneous coronary intervention (PCI) or coronary artery bypass grafting [20]. For patients with chronic heart failure, temporary VAD support may result in bridging to transplant, recovery of myocardial function, or implantation of a long-term LVAD. The usual goal of temporary postcardiotomy support is to allow time for recovery from the insult of cardiotomy with cardiac arrest. However, the mortality in this group is high, and some will eventually receive a long-term LVAD or heart transplant. Patients with myocarditis or peripartum cardiomyopathy usually require support that ranges in duration from many weeks to a few months until recovery is adequate, which usually requires the use of an intermediate to long-term LVAD or biventricular VAD (BiVAD) [21–24]. Patients presenting with cardiogenic shock secondary to valvular or congenital defects will normally undergo corrective surgery after stabilization.
Once temporary VAD support is initiated and hemodynamics are stabilized, the potential for myocardial recovery is assessed frequently, with the goal of keeping the support duration as minimal as possible. Bleeding, infection, and thromboembolic complications during VAD support contribute considerably to morbidity and mortality. These complications, along with severe ischemia during the acute phase of cardiogenic shock, often lead to multiple organ dysfunction. Aggressive supportive medical therapy and normal hemodynamics may allow for safe weaning and removal of the device. Explant of the VAD is considered when renal, hepatic, pulmonary, and neurologic functions are adequate and the patient can tolerate VAD weaning. Optimally, dialysis, mechanical ventilation, and inotropic medications have been discontinued or are being used minimally at the time of VAD explant.
Availability and Selection of VADS
Several factors influence the choice of which MCS device is most appropriate for individual patients. Age and size of the patient is an important consideration in selection of cannulas or devices. Most contemporary VAD systems are small and can accommodate the majority of patients needing support. However, small children usually require surgical implantation and the use of cannulas suitable for their vessel size. VAD systems that are deployed by percutaneous techniques provide only left ventricular support, and the maximal flow rate ranges from 2.5 to 5.0 L/min. The percutaneous LVADs require expertise for insertion of a transeptal left atrial cannula or for transvalvular left ventricular positioning of a pump and cannula. Severe peripheral vascular disease may preclude the use of both the TandemHeart and Impella VADs because the cannulas or pump are inserted through the femoral vein and artery. Fluoroscopic guidance for proper placement of the cannula is necessary with most implant procedures being performed in the cardiac catheterization laboratory. The percutaneous VADs avoid surgery and associated complications, but support is limited to the left heart only, and the amount of support is limited to 5 L/min at best. A percutaneous VAD may be optimal for patients with severe coagulopathy or other surgical contraindications. These devices are most of the time used for up to 14 days and are usually converted to longer-term devices when continued support is indicated. Because the percutaneous devices are commonly intended for left heart support, surgical placement of a CentriMag VAD may be necessary for patients requiring biventricular support. Early assessment of right heart hemodynamics (central venous pressure, pulmonary artery pressures, and right ventricular stroke work index) or echocardiography may provide sufficient evidence for univentricular versus biventricular support. Patients with prolonged cardiogenic shock with end-organ dysfunction, and those with postcardiotomy shock, are best supported by a surgically placed biventricular support system because of its ability to unload both sides of the heart and to provide a greater flow capacity.
The availability of VAD systems may determine the type of support utilized. Most academic medical centers with a full range of heart failure treatments usually have the ability to fit the proper type of VAD to each patient. These major medical centers also have appropriately trained personnel for the different types of devices. Patients with cardiogenic shock often present at nonacademic community medical centers that do not have multiple VAD systems and trained personnel. Collaborative networks between community and academic hospitals with a hub-and-spoke VAD program offer advanced therapy to those who are not near the full-service heart failure programs [25–27]. Transfer of patients in cardiogenic shock to higher levels of care must be done in a timely fashion before end-organ dysfunction becomes irreversible.
Hub-and-spoke VAD networks between community hospitals and academic medical centers need to have dedicated personnel who are in frequent communication. Specialized transport teams with appropriate personnel and technologies have an important role in the successful transfer of patients with cardiogenic shock [28]. The hub hospital or VAD center must have an available specialist to discuss the care and transport of patients and then, be prepared to provide advance care upon receipt of the patient. The spoke hospital or community center must provide prompt inotropic and IABP support or a higher level of circulatory assist to stabilize hemodynamics for preservation of organ function. Importantly, the spoke hospital must rapidly assess comorbidities, patient viability, and financial issues before transporting the patient to another institution [25]. Multiple transfers of patients with low probability of salvage will become a significant financial burden on the hub facility.
Although there are a number of VAD systems that may be used for short-term circulatory support, three of the most recently developed systems are described in Table 8.1. The TandemHeart and Impella devices are used for support of patients with cardiogenic shock and during risk interventional procedures. The CentriMag is a versatile, surgically placed device that is in use in a variety of clinical scenarios.
Table 8.1
Currently available VAD systems for stabilizing patients with severe cardiogenic shock
VAD system | Type of pump | Cannulation | Amount of support (L/min) | Indications |
|---|---|---|---|---|
TandemHeart | Centrifugal flow | Percutaneous (femoral) LA transeptal; femoral artery | Up to 5 | Cardiogenic shock, CPR and high-risk PCI |
Impella 2.5 | Axial flow | Percutaneous femoral insertion; cannula tip in LV, pump in ascending aorta | Up to 2.5 | High-risk PCI |
Impella 5.0 | Axial flow | Surgical femoral insertion; cannula tip in LV, pump in ascending aorta | Up to 5.0 | Cardiogenic shock and high-risk PCI |
CentriMag | Centrifugal flow | Varies; LA or LV to ascending aorta or femoral artery for LVAD. RA to PA for RVAD | Up to 10 | Postcardiotomy |
TandemHeart
The TandemHeart system (CardiacAssist, Inc., Pittsburgh, PA) provides circulatory assist by pumping oxygenated blood continuously from the left atrium to the femoral artery (Fig. 8.2). The goal of support is to reduce cardiac work, oxygen demand, and left ventricular filling pressure. The system consists of a transeptal inflow cannula (Fig. 8.3), a centrifugal flow pump (Fig. 8.4), an outflow cannula (Fig. 8.3), and a bedside control console (Fig. 8.5). The pump can generate up to 5 L/min of blood flow with an impeller speed range of 3,000–7,000 rpm. During support, the pump is positioned near the cannulation sites and usually is secured to the anterior right thigh. Cannulation is usually performed in the cardiac catheterization laboratory with fluoroscopic guidance, or echocardiography may be used in other hospital settings.
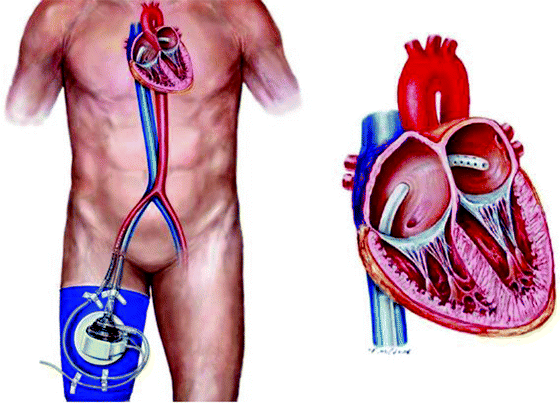


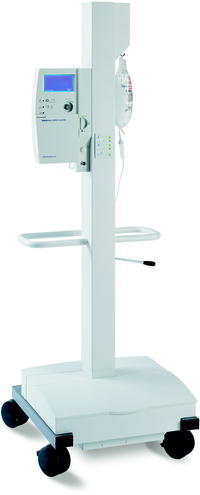

Fig. 8.2
The TandemHeart ventricular assist device (VAD) with view of pump and cannula positioning (left) and transeptal inflow cannula tip in the left atrium (right)

Fig. 8.3
Cannulas for TandemHeart support: transeptal cannula (right), femoral outflow cannula (middle), and two-stage dilator for dilating the opening in the atrial septum

Fig. 8.4
The TandemHeart centrifugal flow blood pump is normally placed on the patient’s thigh during support

Fig. 8.5
The TandemHeart bedside control console is used to adjust the pump’s impeller rotation and to provide a continuous infusion of heparinized glucose solution to the pump
With properly trained personnel, support with the TandemHeart system can be initiated in less than 30 minutes of arrival to the catheterization laboratory [29]. The unique feature of the TandemHeart system is the placement of the 21-F polyurethane inflow cannula into the left atrium. From a femoral vein, a septal puncture is performed with a Brockenbrough needle passed through a Mullins sheath into the right atrium. A 0.035-inch pigtail guidewire is introduced into the left atrium, and a two-stage (14-F–21-F) dilator is used to expand the opening in the atrial septum. The tip of the inflow cannula is passed over the wire into the left atrium; this cannula then is attached to the inflow connector of the pump. A 15-F or 17-F cannula is placed in the contralateral femoral artery and connected to the outflow connector of the pump. The pump impeller speed is adjusted with the bedside control console to achieve the desired flow rate. The console monitors pump function and provides audio and visual alerts during abnormal conditions. The console also provides a continuous infusion of heparinized saline to the lower portion of the blood chamber in the pump to prevent clot formation.
The TandemHeart system has been used in a variety of clinical scenarios, but the primary use has been for support of patients with cardiogenic shock [30–34] and during high-risk PCI [35–38]. With modified cannulation techniques, the TandemHeart system has been used as an RVAD and can provide biventricular support in profound cardiogenic shock [39]. When used as a right ventricular assist device (RVAD), cannulation of the right atrium and pulmonary artery is accomplished through the right internal jugular and femoral veins [40]. The TandemHeart system has also been used for support during cardiac surgery and for postcardiotomy failure [41–43]. Direct surgical cannulation may also be employed with the use of shorter cannulas and flow rates up to 8 L/min.
The early randomized clinical trials comparing support between the IABP and TandemHeart system have shown that hemodynamic parameters are consistently better with TandemHeart support; however, 30-day mortality rates were not different [44, 45]. However, in a recent single-center report of TandemHeart use in 117 patients with severe refractory cardiogenic shock, with nearly 50 % in cardiac arrest and predictive mortality of more than 90 %, improvements in blood pressure, end-organ function, venous oxygen saturation, urine output, and lactic acid levels resulted in a 6-month survival rate of 45 % [46]. In another report involving 22 patients with severe cardiogenic shock supported as a bridge to decision, 34 % survived and underwent device explant, transplant, or implantation of a long-term LVAD [47]. The time from the onset of cardiogenic shock to the initiation of support appears to be an important factor in determining outcome. Patients with cardiogenic shock who are successfully stabilized have therapeutic options that may result in long-term survival. Few patients will recover myocardial function without revascularization or corrective surgery. After a period of support and following more definitive therapy, some patients may continue to have good organ function, but poor cardiac function may be suitable for long-term LVAD support [32, 47, 48]. Prolonged TandemHeart support or conversion to an implantable LVAD or BiVAD may be necessary in cases of acute fulminate myocarditis or peripartum cardiomyopathy [49, 50].
Device-related complications, including bleeding from the cannula insertion site, persistent patent foramen ovale, limb ischemia, and thromboembolism, are observed, but these risks do not preclude the use of the TandemHeart system in a population of patients who are facing imminent death [41]. Persistent patent foramen ovale following inflow cannula removal has not been a significant complication [36, 44]. The TandemHeart is the best percutaneous MCS device in cases of ventricular septal defect (VSD) despite the potential for a left to right shunt. Since the inlet cannula is in the left atrium, the blood is aspirated into the cannula prior to reaching the left ventricle and thus avoiding the RV unsaturated blood to be mixed across the VSD. The TandemHeart is contraindicated in patients with severe peripheral vascular disease that prevents cannulation of the femoral vessels [51]. Because the inflow cannula is passed through the inferior vena cava and right atrium, use of this device is contraindicated with the presence of a caval filter. Bleeding from the cannula insertion site results from the requirement to anticoagulate patients during support. Patients are confined to bed and require sedation to prevent dislodgement of the inflow cannula into the right atrium.
Impella
The Impella system (ABIOMED Inc., Danvers, MA) is a catheter-mounted continuous-flow pump and cannula that aspirates blood from the left ventricle into the ascending aorta. A small axial-flow blood pump mounted on the end of a catheter is positioned in the ascending aorta, with its cannula placed across the aortic valve and the tip within the left ventricular cavity. The amount of flow through the pump is determined by the rotor speed, preload (left ventricular pressure), and afterload (aortic pressure). There are two versions of the Impella device; the 2.5 and the 5.0, which are designations indicating the pump’s maximum flow rate. The 2.5 version has a 12-F diameter cannula and is inserted percutaneously from the femoral artery (Fig. 8.6). The Impella 5.0 device has a 21-F diameter cannula with versions for femoral insertion (Fig. 8.7) or insertion directly into the ascending aorta through a sternotomy (Fig. 8.8).
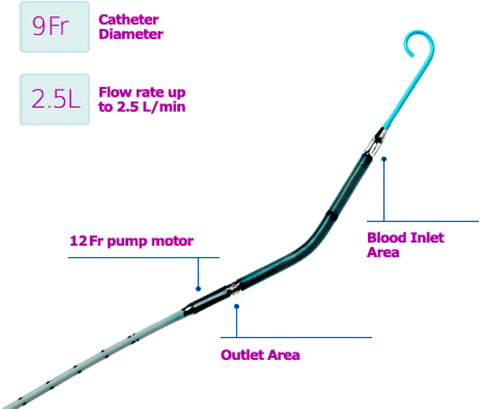
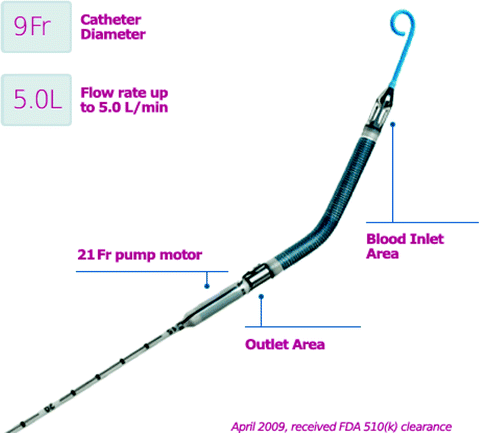
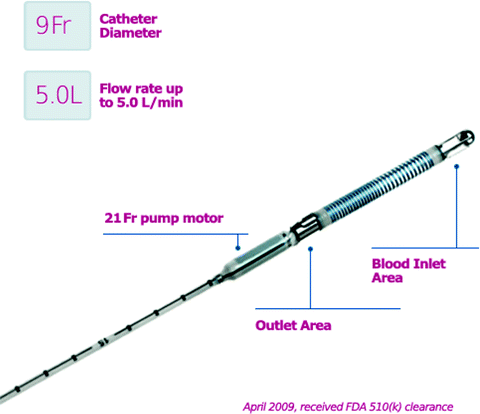

Fig. 8.6
The Impella 2.5 cannula is inserted over a wire via the femoral artery and retrograde through the aorta and across the aortic valve. The blood inlet is within the left ventricle, and the pump outlet is in the ascending aorta

Fig. 8.7
The Impella 5.0 cannula is inserted over a wire via the femoral artery and retrograde through the aorta and across the aortic valve. The blood inlet is within the left ventricle, and the pump outlet is in the ascending aorta

Fig. 8.8
The Impella 5.0 direct (LD) version is inserted through a graft on the ascending aorta with the tip within the left ventricle and the pump in the ascending aorta
Insertion of the Impella 2.5 percutaneous device is normally performed in a cardiac catheterization laboratory with fluoroscopic guidance. This device is used for support of patients with cardiogenic shock or during high-risk PCI. The Impella 2.5 device is inserted percutaneously via the femoral artery through a 13-F sheath. Then a guidewire is passed through the aorta and into the left ventricle, followed by insertion of the pump over the wire until the J-tipped portion enters the left ventricle. Proper placement of the pump is also guided by observation of the pressure waveform, which is detected in the cannula near the pump. The dual-pressure sensor detects the pressure within the cannula and on the outer surface of the cannula. When the cannula crosses the aortic valve, the diastolic pressure within the cannula decreases greatly, indicating entry into the left ventricle, whereas the pressure on the outer portion of the cannula continues to record aortic pressure. Before insertion, the catheter is connected to a bedside console for monitoring and control of the pump speed. A seal within the pump must be continuously purged with a solution of glucose and heparin to prevent clot formation. The bedside console is battery operated for patient transport. Patients must remain supine during support due to the presence of the catheter in the femoral artery.
The Impella 5.0 LD is used intraoperatively to support patients during beating-heart surgery or for postcardiotomy cardiogenic shock. The 21-Fr device is inserted through a graft anastomosed end to side on the ascending aorta. The cannula crosses the aortic valve with the pump just above the valve. If this technique is employed for postoperative support, reoperation is necessary to remove the device and the graft on the aorta.
The Impella 2.5 device has been used for short-term support of patients with cardiogenic shock and during high-risk PCI. Contraindications for the use of this device include the presence of a mechanical aortic valve, severe peripheral vascular disease, and severely calcified aortic valve. Hemodynamic indications for use are a cardiac index <2.0 L/min/m2, arterial blood pressure <90 mmHg and a pulmonary capillary wedge pressure >18 mmHg, and heart failure that is potentially reversible. There are numerous reports of successful support in patients with postcardiotomy low-cardiac output [52–55], myocardial infarction with cardiogenic shock [33, 56–59], acute myocarditis [60, 61], and severe allograft rejection [62, 63]. The Impella device has been used also to stabilize patients with decompensated chronic heart failure who then undergo heart transplant or implantation of a long-term LVAD [33]. In randomized controlled trials, comparison of the Impella 2.5 and the IABP in patients with cardiogenic shock has shown that the Impella device provides better hemodynamics during support, but there is no difference in 30-day mortality [64]. Support during high-risk PCI is safe and provides adequate hemodynamic support, but superiority over IABP support has not been demonstrated in a randomized trial [65]. Since the Impella 2.5 device provides the maximal flow of 2.5 L/min, it has little benefit for patients in severe refractory cardiogenic shock.
CentriMag
The CentriMag Blood Pumping System (Levitronix, Waltham, MA) utilizes a centrifugal flow pump with a magnetically levitated impeller (Fig. 8.9). The impeller is raised away from the pump housing and rotates by magnetic force generated by the motor. This type of impeller rotation improves biocompatibility by eliminating heat from friction, and there is no wear of the moving components. The CentriMag system comprises the pump, an electromagnetic motor, an ultrasonic flow probe, and an external control console. The 3/8-inch diameter inlet and outlet connections accommodate use of standard perfusion tubing. The surgeon determines the cannulas appropriate for the type of support and vessel size. This system is implanted surgically through a sternotomy but also has been adapted for percutaneous use with an oxygenator [66]. Surgical cannulation for univentricular or biventricular support is accomplished with inflow cannulas placed in the left and right atria and with the outflow cannulas placed in the ascending aorta and main pulmonary artery (Fig. 8.10). The maximum flow rate generated by the CentriMag device is 10 L/min at an impeller speed of 5,500 rpm. Blood flow rate is determined using an ultrasonic flow probe attached to the tubing near the pump. The external control console provides a display of impeller speed and pump flow rate and provides audible and visual alerts for abnormal conditions (Fig. 8.11). The control console can be battery operated to provide uninterrupted support during patient transport. Unlike the VAD system with femoral cannula placement, the cannulas used with the CentriMag system may be externalized through the chest or abdominal wall and fixated to allow patient mobility [67, 68].
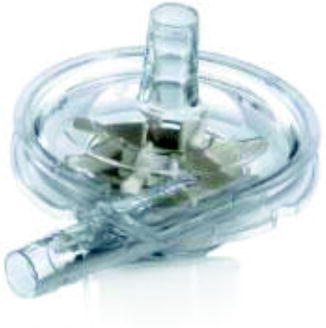
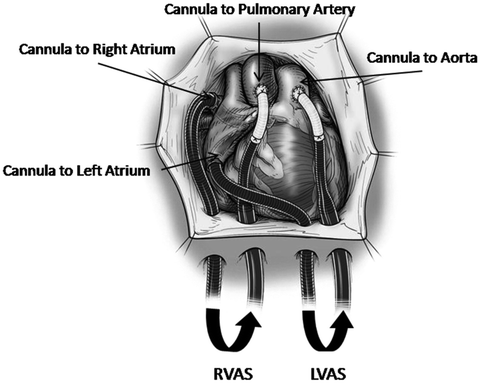

Fig. 8.9
The CentriMag blood pump has a polycarbonate housing with a rotating impeller that is suspended and rotated by magnetic force (Image courtesy of Thoratec Corporation)

Fig. 8.10




The usual cannulation for biventricular assist with the CentriMag device. For left ventricular assist, the inflow cannula is placed in the left atrium, with the outflow cannula in the ascending aorta. For right ventricular assist, the inflow cannula is placed in the right atrium, and the outflow cannula is in the main pulmonary artery (Image courtesy of Thoratec Corporation)
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


