INTRODUCTION
Aortic stenosis (AS) is the most common form of acquired valvular heart disease in Westernized nations and its prevalence increases with age. The natural history of untreated
AS portends a grave prognosis with 1- and 5-year survival rates of 60% and 32%, respectively. Currently, the only effective treatment for patients symptomatic from
AS is surgical aortic valve replacement (AVR). In the ideal candidate, the estimated mortality from surgical
AVR performed by a skilled operator should be <3%. Historically, the rate of mortality and postoperative complications for surgical
AVR have climbed rapidly after the eighth decade of life and in those with multiple pre-existing conditions and comorbidities, such as depressed left ventricular (
LV) function, associated coronary artery disease (CAD), prior cardiac surgery, renal insufficiency, peripheral vascular disease, and chronic lung disease. Because of this increased perioperative risk in the elderly and often frail patient, as many as 30% of patients diagnosed with critical
AS never get referred to a surgeon.
First introduced nearly a decade ago, transcatheter
AVR (
TAVR) has been established as a safe and effective alternative to
AVR in patients previously considered “high risk” or inoperable due to prohibitive predicted preoperative mortality. There are currently two transcatheter heart valve (
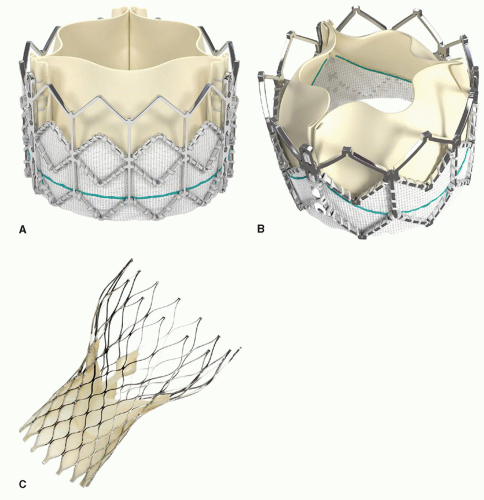
THV) replacement devices approved for use in the United States, each using a unique platform for delivery and having individual nuances regarding patient workup, clinical management, and device deployment. The Edwards Lifesciences (Irvine, CA) SAPIEN
TM THV system (transfemoral: Retroflex 3 [RF3] and newer generation XT; transapical: Ascendra) are balloon expandable devices consisting of bovine pericardial valve leaflets attached to stent platforms placed in the subcoronary position. The transfemoral SAPIEN RF3 is currently the only
THV that is FDA approved for commercial use in the United States for inoperable patients. In contrast, the Medtronic (Minneapolis, MN) CoreValve
TM Revalving system is a self-expanding, nitinol cage with porcine leaflets, which seats in the
LV outflow tract and spans through the aortic valve to above the coronary ostia. Both
THV systems will be discussed in this chapter. In addition, several next generation devices are in various stages of development and worthy of brief comment.
RISK STRATIFICATION, PATIENT SELECTION, AND SCREENING
Despite the mismatch between incidence of critical aortic valve disease and surgical referral for AVR,
TAVR is currently only available to the highest risk patient population with symptomatic, calcific severe
AS (see
Table 50.1 for accepted echocardiographic criterion for severe AS). who are currently without a surgical option or whose risk of mortality exceeds 15%. Hence, the typical
TAVR patient is in the top 10% for operative mortality with a Society of Thoracic Surgery (STS) PROM score typically >10 and having a corresponding Logistic European System for Cardiac Operative Risk Evaluation score (EuroSCORE) of >30. Data from multiple well-designed prospective randomized trials have demonstrated that
TAVR, although carrying a higher incidence of stroke, is a viable alternative to surgical
AVR in patients with this extreme predicted operative risk. However, due to unproven long-term durability and efficacy,
TAVR has not been offered in lower risk patients and further study is needed to determine if
TAVR will be appropriate in these populations.
Risk assessment for
TAVR is not solely for the purpose of evaluating periprocedural mortality, however. A more important function of risk assessment in this population is to better understand the natural history of patients treated with
TAVR. It is well documented that surgical models of risk fail to capture the true risk of patients with severe comorbidity while overestimating risk in elderly patients, and offer little validation in the current
TAVR population. Furthermore, models of surgical risk provide no information regarding important clinical variables such as cost-effectiveness, long-term morbidity, and noncardiac mortality, which are all vital in determining which patients should be treated with
TAVR. For that reason, risk assessment must take into account both traditional methods of risk scoring, such as the STS and EuroSCORE systems, which
are based on historical data of outcomes from coronary and valvular surgery, as well as newer, nontraditional methods, such as frailty scoring and adjusted incremental risk. A frailty index used at our institution has proven to be invaluable to our assessment and its components are described in
Table 50.2. In addition, taking into account factors not integrated in the STS or EuroSCORE, such as pulmonary hypertension, cirrhosis, and dementia, can weigh heavily in final patient selection. In general, any patient with a life expectancy of less than 12 months due to a noncardiac condition or with such severe medical comorbidity (i.e., extreme frailty, moderate-to-severe dementia, etc.) that will prevent meaningful recovery should not be offered
TAVR. A comprehensive list of relative exclusion criteria pooled from existing clinical trials is provided in
Table 50.3.
Appropriate patient selection and screening is crucial to maximize the chance of procedural success. Once a patient has been deemed a nonoperative or high-risk surgical candidate, the primary determinants of
TAVR eligibility initially focus on aortic annulus sizing and anatomy and route of device delivery.
Table 50.3 lists the current annulus sizing criteria for patients eligible for both the SAPIEN and Core Valve
THV. Patients with extremely small or large annuli (<18 or >25 mm) may face a higher risk of aortic/ventricular rupture or severe paravalvular leak (
PVL), respectively, with current devices, and should be avoided for
THV use until suitable devices are available. There are a number of anatomic considerations that may also preclude
TAVR success: (1) noncalcified valves may not provide adequate annular support for an expanded valve stent and have not been studied, (2) patients with unicuspid or congenital bicuspid aortic valves may not allow proper
THV stent expansion, (3) patients with leaflet thrombus or vegetation are not
THV candidates, (4) placement of a
THV in the presence of low-lying coronary ostia, bulky calcified leaflets, or an effaced sinus of Valsalva may result in coronary obstruction, and careful consideration for coronary vessel access must be made prior to
THV deployment, and (5) patients with severe aortic insufficiency should be evaluated with caution. Inadequate characterization of this complex anatomy may lead to serious procedural morbidity; complications have included valve migration and embolization, annulus rupture, aortic dissection, and severe
PVL. Many of these complications can be avoided with careful planning.
The delivery of
THV may be performed by either a transfemoral or transapical approach. The transfemoral approach consists of retrograde percutaneous delivery through femoral artery access, while a transapical approach requires a mini-left thoracotomy for
LV apical exposure, followed by percutaneous antegrade delivery via the
LV apex. Advantages to both routes exist but patient considerations usually dictate the approach. In patients with suitable femoral access, a transfemoral approach may be preferable to transapical, as pain and recovery from mechanical ventilation may be reduced. However, in patients with poor vascular access, the transapical approach offers a safe and direct means for device delivery. To date, both access routes have shown equivalent outcomes versus surgical AVR. Vascular size criterion for transfemoral access for both the Edwards SAPIEN and Medtronic CoreValve systems, as well as inclusion/exclusion factors, is described in
Table 50.4.
Successful alternative methods of
THV delivery have been reported as off-label use and/or outside the United States. These routes, such as direct-aortic (through a hemi-sternotomy or right third intercostal space parasternal exposure), subclavian or axillary artery cutdown, or via abdominal aorta conduit, present separate technical challenges in terms of surgical exposure and device deployment, and should only be performed when necessary and by highly qualified operators. To date, placing a
THV into a bioprosthetic valve has been performed worldwide with some success in isolated cases, but full evaluation of these techniques have not been documented and are beyond the scope of this chapter.
PREOPERATIVE EVALUATION
Preoperative testing for patients being considered for
TAVR is comprehensive, as
both eligibility for valve placement from an anatomic standpoint must be determined, as well as testing for fitness to tolerate the procedure in this sick cohort of patients.
Evaluation of the aortic valve, aortic root, and peripheral vasculature provides the necessary clinical information to confirm eligibility for
TAVR. The echocardiogram is the primary modality to document the severity of
AS and size the aortic valve annulus (see
Table 50.1 for echocardiographic description of severe AS). Valuable information regarding the calcium severity of the leaflets, valve type (uni-, bi-, or tricuspid), annulus to coronary ostia height, and leaflet length and bulkiness may also be obtained. Note that measurement in the parasternal long axis views and during systole most accurately size the annulus (
Figs. 50.4A and B). Preoperative transthoracic echocardiography may be sufficient for most patients, but transesophageal echocardiography (TEE) should be utilized in the event of any discrepancy or concern. Low gradient in the presence of low-ejection fraction is frequently encountered in high-risk and elderly patients; in these cases, dobutamine stress echocardiography is mandatory to evaluate contractile reserve and candidacy for
TAVR.
Multidetector computed tomography (
MDCT) angiography (CTA) of the chest, abdomen, and pelvis with iliofemoral runoff is performed, detailing the anatomy of the aorta and aortic root, with special attention given to aortic root size, aortic tortuosity and angulation (especially at the arch), calcification, areas of stenosis, and adequacy of peripheral vessels for the transfemoral approach. Iliac angiography or computed tomography (CT) reconstruction of peripheral vessels is mandatory to confirm vessel adequacy for the transfemoral approach (see
Table 50.5 for minimum vessel size for
TAVR). Aortography may be used selectively as an adjunct to echocardiography and CT scanning. Iliac intravenous ultrasound can be substituted for CTA scan to avoid intravenous dye administration in patients at risk of renal failure.
A standard right and left heart cardiac catheterization should be performed to evaluate the presence of pulmonary hypertension and any concomitant
CAD. Depending upon the severity, certain coronary lesions may need to be addressed prior to consideration for
TAVR. The use of systemic anticoagulation or platelet inhibitors should not affect
TAVR candidacy, although
TAVR within 1 month of bare-metal stent placement and within 6 months of drug-eluting stent placement is not recommended. Finally, other comorbidities, such as advanced pulmonary disease, chronic renal insufficiency, and ongoing gastrointestinal disease should be addressed as part of a standard preoperative workup.
 SURGICAL TECHNIQUE
SURGICAL TECHNIQUE