Ross Procedure
John D. Oswalt
The Ross procedure, first performed by Mr. Donald Ross in 1967, is the replacement of the aortic valve with the autologous pulmonary valve. It is considered the valve of choice for aortic valve replacement in children. It is also ideal for replacement in younger patients, athletes, women of child-bearing age, and patients with endocarditis. The operation since 1967 has evolved with better standardization of techniques and in multiple long-term studies has produced similarly excellent results.
 INDICATIONS
INDICATIONSPatients with a life expectancy of more than 20 years are candidates for the Ross procedure. Likewise, patients who cannot safely take or have a lifestyle not desirous of permanent anticoagulation are candidates for the procedure. Athletes whose sport requires extended periods of highly elevated heart rates will also see better performance with an autograft than a mechanical valve. Over the last 20 years, the Ross procedure has proven highly effective for aortic endocarditis. The autograft, with the flexibility of tissue and used as a root, allows for increased cure of the infection and has better longevity than other valve choices. Initially, discrepancy in aortic annulus to pulmonary annulus size was a contraindication. With improved techniques of dealing with the enlarged aortic annulus, reduction and fixation in a size suitable to the recipient, the Ross procedure is now performed in these patients. In the pediatric age group, the Ross procedure can be combined with the Konno procedure (myotomy and myectomy), particularly useful in combination with left ventricular outflow tract (LVOT) obstruction with aortic stenosis. An extension of right ventricular outflow tract (RVOT) muscle can be harvested with the autograft to fill in the myotomy for the enlargement of the LVOT. The viability of this graft then allows for continued growth with the child.
CONTRAINDICATIONS
Patients with Marfan’s or other genetic disorders known to affect fibrillin or elastin of the aortic valve are not candidates for the Ross procedure because the pulmonary valve is likely to be affected by the same disease process. This includes patients with bicuspid aortic valve associated with root enlargement. Patients afflicted with significant immune-complex disease (juvenile rheumatoid arthritis, lupus erythematosus, and active rheumatic heart disease) as the etiology of their aortic valve disease have had early failure or degeneration of the pulmonary autograft valve. These patients are not considered candidates for a Ross procedure. In general, patients who fall into these categories have had early failure of the autograft through dilatation of the root into an aneurysm and/or development of aortic insufficiency.
 SURGICAL TECHNIQUE
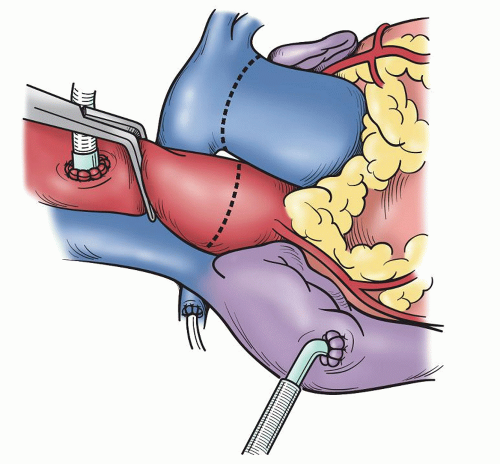
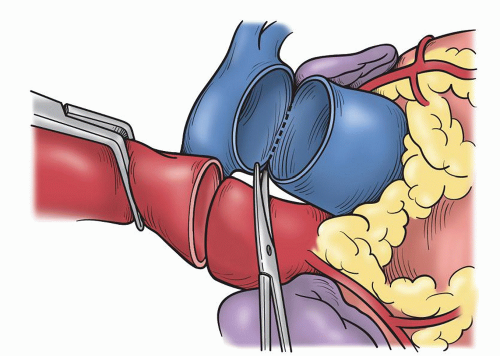
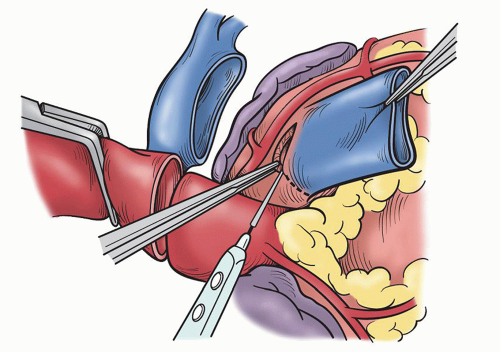
SURGICAL TECHNIQUEA standard median sternotomy is recommended, as adequate exposure of the great vessels and roots is mandatory for safe harvesting of the autograft and implantation of the coronary ostia. The operation then proceeds as for an aortic valve replacement. Cannulation high on the aorta is done in case remodeling or resection of some of the ascending aorta is necessary. Either bicaval venous cannulation or two-stage single venous cannulation is acceptable; however, care must be taken to avoid air lock in the venous return when the RVOT is opened. Antegrade cardioplegic and aortic sump line is placed as well as retrograde coronary sinus cannula for intermittent or continuous retrograde blood cardioplegia. Manipulation of this cannula can be facilitated by the echoanesthesiologist without having to open the atria. A left ventricular sump is placed via the right superior pulmonary vein (Fig. 49.1). As cardiopulmonary bypass is begun, dissection between the great arteries is performed until the take-off of the right pulmonary artery is seen. Systemic cooling is accomplished to 32°C to 34°C. With aortic clamping antegrade followed by retrograde blood cardioplegia is delivered for complete arrest. This is facilitated with saline slush in the pericardial well. Initially, the autograft is harvested by making a transverse arteriotomy proximal to the right pulmonary artery take-off (Fig. 49.2). Inspection of the valve leaflets is done to rule-out a deformity (bicuspid or fused) in the pulmonary valve (Fig. 49.3). A bicuspid pulmonary valve should not be used. Finding this, one would abort the Ross and use the backup valve planned with the patient preoperatively. The cryopreserved pulmonary homograft can then be thawed, so it will be ready for implantation later. The harvesting of the autograft is begun posteriorly using the electrocautery. One must dissect close to the pulmonary artery. Caution is taken as the left main coronary artery lies posterior and superior at the base of the pulmonary artery. Dissection is carried down to the level of ventricular muscle (Fig. 49.4). A right-angled clamp is then placed through the opening of the pulmonary valve visualizing the RVOT muscle beneath the annulus. The clamp can then be pushed through the muscle about 3 to 4 mm from the annulus (Fig. 49.5). From this opening, sharp dissection is accomplished with scissors and direct visualization of the annulus. Three millimeters of subannular muscle is taken with the graft. Initial dissection is to the patient’s left to the point where the posterior wall of the RVOT begins. Similarly, the dissection is taken to the patient’s right, again to the posterior wall. Scalloping the underlying muscle is useful always maintaining the same 3 to 4 mm of muscle (Fig. 49.6). The posterior wall is incised with a scalpel and dissection is carried to a depth in the muscle similar to the thickness of the RVOT muscle cut through anteriorly. Embryologically, the RVOT is separate
from the septal muscle and this plane can be seen as one dissects directly posterior. When this plane is reached, care must be taken to avoid the first septal perforator which runs in this layer. The most common course of the artery is from the anterior descending coronary to the middle papillary muscle of the tricuspid valve, which is easily visualized. The angle of dissection is changed to be more horizontal and is carried craniad until the level of the previous posterior dissection is reached. This is the enucleation of the valve. Caution around the sinuses is important as too close dissection may injure the sinus wall. The valve can then be inspected for injury and then placed in the pericardial well for safe keeping. This harvested valve can later be used as a subcoronary (scalloped) valve or as a root.
from the septal muscle and this plane can be seen as one dissects directly posterior. When this plane is reached, care must be taken to avoid the first septal perforator which runs in this layer. The most common course of the artery is from the anterior descending coronary to the middle papillary muscle of the tricuspid valve, which is easily visualized. The angle of dissection is changed to be more horizontal and is carried craniad until the level of the previous posterior dissection is reached. This is the enucleation of the valve. Caution around the sinuses is important as too close dissection may injure the sinus wall. The valve can then be inspected for injury and then placed in the pericardial well for safe keeping. This harvested valve can later be used as a subcoronary (scalloped) valve or as a root.
SUBCORONARY IMPLANTATION
Most surgeons now use one of the two techniques for the implantation of the autograft. The subcoronary technique, Mr. Ross’ original technique, uses a hockey-stick aortotomy. The aortotomy is started 2 to 3 cm above the right coronary ostium and angled down into the noncoronary sinus. The aortic valve is excised and the annulus sized. The autograft is then trimmed of excess epicardial fat and tissue, and the subannular muscle is trimmed to 2 to 3 mm. The sinuses of what will be the left and right sinus of the autograft are excised leaving 2 to 3 mm of arterial wall above the annulus. The distal artery is trimmed to just above the commissures. This leaves the noncoronary sinus intact. Implantation is accomplished by interrupted 4-0 polypropylene sutures or in running single or triangulatedrunning suture. The triangulation is done by placing three separate sutures through the aortic annulus and in the subannular autograft at the nadir of the cusp. Interrupted sutures are then placed at 2 mm separation between the triangulated sutures. Some surgeons will reinforce this proximal suture line with a strip of autologous pericardium. If a running triangulated suture technique is used, each suture can then be run to the commissures. Similarly, a single running suture can be done from the nadir of the left cusp (beneath the left coronary ostium) and run first counterclockwise to the right noncommissure followed then by the other end of the suture run clockwise to meet the suture at the right noncommissure. Some surgeons facilitate the exposure by inverting the autograft valve into the LVOT. As the muscle of the autograft was somewhat scalloped with the harvesting, the line of the anastomosis will follow the natural annulus. With the proximal anastomosis completed, the distal implant is accomplished with a running 4-0 polypropylene suture. The right–left commissural post is tacked to the sinus wall 3 to 5 mm above the existing native commissure. It is important to have the post in this suspended position as laxity will result in prolapsing of the leaflets. The noncoronary sinus maintains the relation of its connected commissural posts at 120 degrees. Tacking of the other posts is facilitated by leaving the noncoronary sinus intact. The suture line proceeds in scallop manner beneath the coronary ostia attaching the autograft to the sinus walls in a hemostatic manner. The noncoronary sinus is tacked to the remaining aortic wall to remove any free space behind it. Closure of the aorta then incorporates aorta to autograft in a manner as to not buckle
the noncoronary sinus of the autograft. Advantages of this implantation technique include not having to reimplant the coronary ostia and avoidance of late sinus dilation as may be seen with the root technique. Disadvantages include inability to perform if there are dilated sinuses and inability to do if calcifications exist in the sinus walls prohibiting the suturing in the wall of the sinus. Groups with long-term results using this technique have achieved similar results with those surgeons using the root implantation technique.
the noncoronary sinus of the autograft. Advantages of this implantation technique include not having to reimplant the coronary ostia and avoidance of late sinus dilation as may be seen with the root technique. Disadvantages include inability to perform if there are dilated sinuses and inability to do if calcifications exist in the sinus walls prohibiting the suturing in the wall of the sinus. Groups with long-term results using this technique have achieved similar results with those surgeons using the root implantation technique.
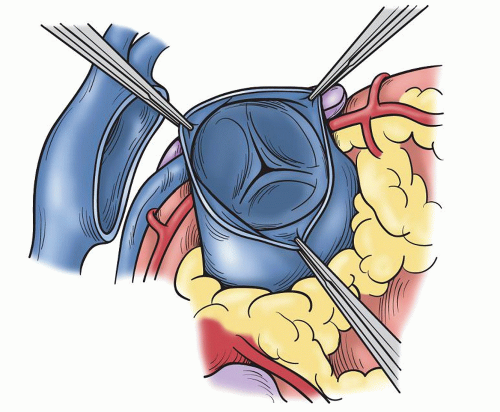 Fig. 49.3. The normal trileaflet pulmonary valve with three equal sinuses and no significant fenestrations or other abnormalities of the leaflets. |
ROOT IMPLANTATION
The root implantation technique has become the most popular for insertion of the pulmonary autograft. It is easier to insure a competent aortic valve as no manipulation of the commissural posts is required. Since beginning the use of the Ross procedure, we have exclusively used this technique. Patients with dilated sinuses, dilated sinotubular junction, true bicuspid valve with 180-degree coronaries are all candidates for a root replacement. Cannulation is the same for the root replacement as with the subcoronary technique. Bypass and autograft harvesting are likewise the same. Preparation of the autograft is different as the adventitia is left intact but the muscle below the annulus is trimmed to 2 to 3 mm. The aorta is transected at the sinotubular junction with dissection of tissue behind the aorta to enter the transverse sinus. The aortic valve is excised and the annulus measured. Each coronary ostium is excised with a portion of aorta creating “buttons” that will be reimplanted later into the autograft. Mobilization of each ostium is important to allow for proper reimplantation. The noncoronary sinus is left intact and not excised. This will be used as reinforcement to the root in patients who have a dilated annulus, dilated sinuses, or sinotubular junction. If the patient has aortic insufficiency or has an annular size larger than the normal aortic size for the patient’s body surface area, an annular reduction and fixation is accomplished using a double row of 2-0 polypropylene placed in a intra-annular plane and brought to the outside of the noncoronary cusp for tying through a pledget. This double row of suture run in pursestring manner can then be tightened over a sizer to achieve the desired annular size for the specific patient (Fig. 49.7




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree