Thromboembolism
Andrzej Szuba
Izabela Gosk-Bierska
Richard L. Hallett
Venous Thromboembolism
Deep vein thrombosis (DVT) and its primary complication—pulmonary embolism (PE)—are the two elements of venous thromboembolism (VTE), and epidemiological considerations regarding VTE always, to a greater or lesser extent, apply to both diseases.
Incidence
The incidence of VTE in the United States may be more than 100 per 100,000 patients; encompassing about 2 million new cases annually (1,2). About 30% of new VTE patients die within 30 days from the diagnosis, and 20% suffer sudden death due to PE (3). Long-term consequences of VTE include recurrent VTE, development of post-thrombotic syndrome, and pulmonary hypertension.
Frequency estimates of fatal PE are much less reliable than of DVT, as diagnosis of PE is difficult, and postmortem studies are selective. Only one third of PEs are recognized antemortem (4). VTE, therefore, remains a serious socioeconomic problem (5).
Rudolf Virchow described three main factors responsible for activation of coagulation process and formation of blood clot: changes in the vessel wall, changes in the blood
components, and venous stasis, later called Virchow’s triad. After more than 100 years, all identified prothrombotic risk factors still fall into one of the original Virchow categories (Table 9-1):
components, and venous stasis, later called Virchow’s triad. After more than 100 years, all identified prothrombotic risk factors still fall into one of the original Virchow categories (Table 9-1):
Table 9.1 Prothrombotic Risk Factors | |
|---|---|
|
Vessel wall factors: trauma (iatrogenic, accidents), surgery, sepsis, varicose veins, post-thrombotic syndrome
Blood component abnormalities: inherited and acquired thrombophilia (Fig. 9-1), hyperviscosity syndromes, surgery and the postoperative period, pregnancy, use of oral contraceptives, cancer, nephrotic syndrome, trauma, burns, infections
Venous stasis: due to immobilization (critically ill patients, surgery), congestive heart failure, chronic venous insufficiency, obesity, hyperviscosity syndromes, and the postpartum period and pregnancy
Any disturbance in the fine balance between coagulation and fibrinolysis will result in either a procoagulant state or a bleeding diathesis.
Inherited Thrombophilia
Inherited Thrombophilic States
Hereditary predisposition to vascular thrombosis due to genetic alterations appears to be quite common. In a study of New York newborns, mutations of four genes related to thrombophilia were found in 17.5% of tested newborns (6). Inherited risk factors for thromboembolism (antithrombin III deficiency, protein C deficiency, protein S deficiency, factor V Leiden, prothrombin 20210A) were found in 40% of patients with spontaneous lower extremity DVT before the age of 45 and in 33% of all other patients with leg DVT (7).
Antithrombin III Deficiency
Antithrombin III (AT III) is a member of the serpin family of proteins, which inactivates serine proteases. AT III inactivates thrombin, active factors III, IX, X, XI, and factor VIIa/TF complex.
Multiple genetic defects can lead to quantitative (type I) or qualitative (type II) AT III deficiency. Homozygous AT III deficiency leads to thromboembolic complications early in childhood (8) and is rarely described. Most patients with AT III deficiency have the heterozygous form. AT III deficient patients (heterozygous) of both the quantitative and qualitative types have AT III activity usually at about 40% to 60% of the normal value. The risk of VTE in these people with AT III deficiency was increased eightfold in one comparative study (9).
Protein C Deficiency
Protein C is activated by a thrombin-thrombomodulin complex. Active protein C is a potent coagulation inhibitor and inactivates factors Va and VIIIa on the platelet surface.
Multiple mutations causing type I or type II protein C deficiency have been found. The risk ratio of VTE in protein C deficient patients was reported as 7.3 (9).
Protein C deficiency is found in 2.3% of unselected patients with VTE (10).
Protein S Deficiency
Factor V Leiden
Factor V Leiden (FVL) is a hereditary defect that is caused by a single point mutation Arg506→Gln of factor V. This renders activated FVL resistant to hydrolysis by activated protein C, thus prolonging its thrombogenic effect.
It is the most common known cause of hereditary hypercoagulability in Caucasians and is present in almost 5% of
people of European descent (13). In unselected patients with VTE, FVL is found in 18.8% of Caucasians (13) but only in 1.4% in blacks (11). Relative risk for VTE was found to be 7 in heterozygotes and 40 to 80 in homozygotes (14).
people of European descent (13). In unselected patients with VTE, FVL is found in 18.8% of Caucasians (13) but only in 1.4% in blacks (11). Relative risk for VTE was found to be 7 in heterozygotes and 40 to 80 in homozygotes (14).
Prothrombin G20210A
The G20210A mutation of the prothrombin gene results in higher gene expression without a change in protein structure (15). The G20210A prothrombin gene allele is present in 2% of healthy Caucasian individuals and in 4% to 8% of patients with VTE (10,16). The risk of VTE in heterozygotes is 1.3 to 3-fold higher than in controls (17,18). This defect was not found in samples of the Indian population (19).
The G20210A mutation is found more frequently in children with central nervous system (CNS) thrombosis and arterial thrombosis (20).
Hyperhomocysteinemia
Elevated serum homocysteine may be caused by genetic and environmental factors.
Homocystinuria occurs in homozygotes for the genetic deficiency of cystathionine β-synthase (CBS). It is a recessively inherited disorder with a reported incidence of 1:650,000 to 1:344,000 (21). Patients with homozygotic CBS deficiency have very high levels of plasma homocysteine (>100 μmol/L) and carry an increased risk of vascular complications (22,23). Heterozygotes for CBS deficiency have a mild elevation of plasma homocysteine but a comparable risk to the normal control group (24). However, some authors suggest an increased risk (25).
Mild to moderate hyperhomocysteinemia may increase risk of DVT (26,27,28) and stroke (29); however, some studies show only a weak effect or no effect at all to elevated homocysteine in reference to the risk of VTE (30). The relationship between hyperhomocysteinemia and arterial occlusive disease is much better documented (31).
One genetic factor that causes mild to moderate homocysteinuria is thermolabile methylenetetrahydrofolate reductase (MTHFR)—a C677T polymorphism of the MTHFR gene (32).
The MTHFR C677T gene polymorphism is found in 13.7% of Caucasians (33). Recent studies on MTHFR gene polymorphism did not prove an increased incidence of venous and arterial thromboembolic disease (30,34). One recent study showed an increased risk of small artery occlusive disease in subjects with C677T MTHFR polymorphism (35).
Lipoprotein(a)
Lipoprotein(a) [Lp(a)] inhibits fibrinolysis by binding to fibrin and other plasminogen substrates (36). Elevated plasma Lp(a) is a well-established risk factor of atherosclerosis (37,38). Lp(a) may also increase the risk of venous and arterial thrombosis; however, the results of published studies are conflicting (39,40,41,42).
Other Risk Factors for Inherited Thrombophilia
Multiple other abnormalities of genetic or undefined origin are postulated to cause thrombophilia. These include elevated plasma levels of coagulation factor VIII (43), especially prevalent in black patients with VTE (11); elevated levels of factors IX, X, XI (44); activated protein C (APC) resistance not related to factor V Leiden (45); heparin cofactor II deficiency (46); histidine-rich glycoprotein elevation (47); TAFI gene polymorphism (48); dysfibrinogenemia (49); dysplasminogenemia (50,51); PAI-1 gene polymorphism (52); and hyperprolactinemia (53).
Multifactor Genetic Thrombophilia
Coexistence of two or more genetic risk factors is not uncommon and significantly increases the risk of venous and arterial thrombosis (54,55).
In pooled analysis of eight studies, double heterozygotes for factor V Leiden and prothrombin G20210A were found in 2.2% of patients with VTE and none in the control group. The odds ratio for VTE in the double heterozygotes was 20.0 in comparison with 4.9 for FVL or 3.8 for prothrombin G20210A only (56).
Acquired Thrombophilia
Acquired Prothrombotic States
Acquired thrombophilia may result from antiphospholipid syndrome (APS), the use of oral contraceptives, acquired deficiencies of natural anticoagulants, hyperfibrinogenemia, or acquired hyperhomocysteinemia. Other acquired risk factors for thrombosis include age, malignancy, immobilization, previous VTE, major surgery, trauma, intravenous (IV) catheters, congestive heart failure (CHF), nephrotic syndrome, diabetes mellitus, vasculitis, hematological hyperviscosity syndromes, inflammatory bowel diseases, infection (HIV, herpes, sepsis), obesity, and pregnancy.
Antiphospholipid Syndrome
APS, being probably the most common autoimmune disorder, is characterized by venous and/or arterial vascular thrombotic complications (including miscarriages) and the presence of autoantibodies against membrane phospholipids
(antiphospholipid antibodies or APAs) in blood. APAs are responsible for the prolongation of clotting time in patients with systemic lupus erythematosus (SLE) and were named lupus anticoagulant. However, the presence of APA was found to predispose to thrombotic complications rather than to bleeding (59).
(antiphospholipid antibodies or APAs) in blood. APAs are responsible for the prolongation of clotting time in patients with systemic lupus erythematosus (SLE) and were named lupus anticoagulant. However, the presence of APA was found to predispose to thrombotic complications rather than to bleeding (59).
APAs occur in 1% to 5% of the population and increase with age (50% of patients older than 80 years have APAs) (60). These antibodies can be detected in 12% to 30% of patients with SLE (61). They are also detected in syphilis, tuberculosis, viral infections (HIV, hepatitis C virus), mycoplasma, and other infections (62,63); however, APAs associated with infections usually do not predispose to thrombotic complications (63). APAs may be present more frequently in patients with cancer and severe alcoholic intoxication (64). Familial occurrence of APA is also reported (65).
APAs form a heterogeneous group of antibodies against complexes of phospholipids and their protein cofactors (66), especially beta2-glycoprotein-I (67,68) and prothrombin (69). The mechanism of the prothrombotic action of APA is not fully elucidated and may include endothelial damage, impairment of fibrinolysis, platelet activation, interference with natural anticoagulants, and possibly other mechanisms (70,71,72).
Preliminary diagnostic criteria were proposed in 1998 in Sapporo, Japan, and published in 1999 (73). The APS diagnosis can be made in presence of one clinical and one laboratory criterion (Table 9-2).
The clinical presentation of antiphospholipid syndrome includes venous thromboembolic disease, arterial thrombosis (often cerebrovascular), mild thrombocytopenia, and recurrent fetal loss.
Secondary APS most often is recognized in patients diagnosed with SLE, while primary APS is diagnosed when no primary associated disease is recognized.
Catastrophic antiphospholipid syndrome (CAS) is a rare, dramatic worsening of APS with symptoms of multiorgan failure caused by widespread thrombosis of the microvascular bed.
CAS mortality rate approaches 50%. Of survivors, 26% develop APS-related thrombotic complications (74).
Antiphospholipid Syndrome Treatment.
Although recognized as an autoimmune disorder, APS does not respond to immunosuppression. Typical treatment is limited to oral anticoagulants, sometimes with low doses of acetylsalicylic acid.
Patients with APS and venous or arterial thrombosis require lifelong oral anticoagulation with an international normalized ratio (INR) for blood clotting time between 3 and 4; however, there is no consensus on this issue (61,75,76). Pregnant women with APS and a history of miscarriages will require anticoagulation with heparin and low-dose acetylsalicylic acid (77,78).
Table 9.2 Antiphospholipid Syndrome—Diagnosis and Clinical Presentation | ||
|---|---|---|
|
There are no data on the benefits of prophylactic anticoagulation therapy in patients with high titers of anticardiolipin antibodies without a previous history of thrombosis (61).
Risk Factors
Age
Immobilization
Immobility, regardless of the reason, increases the risk of VTE as a result of venous stasis. It is an important and easily recognizable risk factor and an indication for VTE prophylaxis in hospital patients (81,82). Immobility due to nonmedical conditions like prolonged air travel predisposes to calf DVT and PE. Asymptomatic DVT may occur in up to 10% of passengers on long flights (83,84); however, only passengers with preexisting thrombophilia may have an increased risk of VTE (85).
Obesity
Smoking
Smoking results in injury to the vessel wall (88) and increases the risk of arterial cardiovascular events. Smoking is a well-known risk factor for cardiovascular arterial thrombotic events, including myocardial infarction (MI) and ischemic stroke, but also might predispose to VTE (89). The risk is probably small and was not found in other studies (80). Smoking increases the risk of arterial and venous thrombosis in women who take oral contraceptives (90,91,92).
Pregnancy and Puerperium
Pregnant women are at a six times higher risk of VTE than nonpregnant women. VTE remains a major cause of morbidity and mortality in pregnancy (93), and the risk of VTE is even higher in the postpartum period (94). Risk of VTE is further increased with obesity, age >35 years, emergency cesarean section, preeclampsia, previous VTE, or the presence of acquired or inherited thrombophilia (94,95,96,97).
Arterial thromboembolism in pregnancy is rare and is related to thrombophilia, vasculitis, and arterial dissection (98).
Previous Venous Thromboembolism
Patients with a history of VTE are at higher risk of recurrent VTE. The increased risk may result from preexisting thrombophilia as well as injury to the vessel wall.
Malignancy
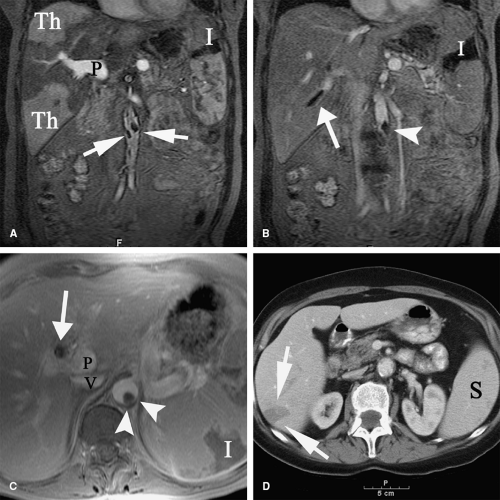
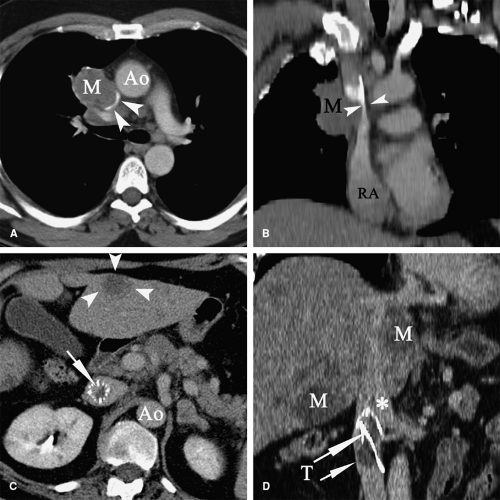
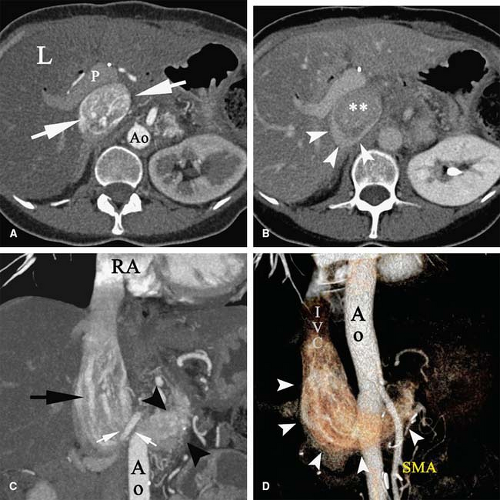
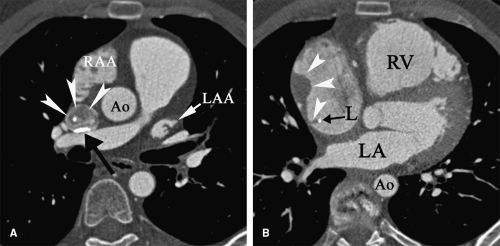
Patients with malignancy are at a higher risk for venous and arterial thrombosis. Vascular thrombosis is a major cause of morbidity and mortality in cancer patients (101,102,103). Breast, colorectal, and lung cancer are frequently associated with vascular thrombosis (Fig. 9-2); the malignancies with the highest risk of thrombotic complications are pancreatic cancer, brain tumors, liver cancer, ovarian and uterine cancer, prostate cancer, and Hodgkin lymphoma (104,105,106,107,108). Increased risk of thrombosis is multifactorial, related to hypercoagulability, vascular injury, and hemodynamic changes in cancer patients. Identified mechanisms of thrombophilia in malignancy include expression of tissue factor and cancer procoagulant on cancer cells (109) and hyperviscosity related to polycythemia vera and/or thrombocytosis (110,111). Cancer infiltration of blood vessels may result in vascular stenosis or occlusion (Fig. 9-3). Cancer therapy may also trigger vascular thrombosis (long-term IV catheters, endothelial injury caused by chemotherapy, radiation injury to the vascular wall) (112,113,114,115).
Infections
Infections may trigger formation of venous or arterial thrombosis, due to systemic activation of the coagulation cascade (118,119,120) or through local injury to the vessel wall per continuitatem from an adjacent infected organ (121,122,123,124).
Certain infectious agents like Chlamydia pneumoniae and HIV are suspected to predispose to VTE; however, the association is yet to be confirmed in larger studies (125,126,127).
Major Trauma
Other Diseases
Multiple medical conditions are associated with an increased incidence of venous and/or arterial thrombosis. Up to 12% of patients with CHF had evidence of PE in autopsy studies (134). Risk of VTE is higher in patients with a low ejection fraction (135). Also, patients with paralysis caused by stroke or spinal cord injury (136) are at risk of thromboembolic complications. Children and adults with nephrotic syndrome suffer more frequently from venous and arterial thromboembolic events secondary to dysproteinemia (low AT III, high fibrinogen) (137,138). Inflammatory bowel diseases are associated with thrombophilia and increased incidence of VTE (139).
Major Surgery
Surgical procedures are considered a risk factor for VTE. However, recent analysis suggests that the frequency of VTE in hospitalized nonsurgical and surgical patients is similar (136). A large retrospective analysis of over 1.6 million surgical
patients revealed an overall frequency of VTE within 3 months after surgery of 0.8%. Increased risk of VTE was associated with age, Latino and Asian/Pacific Islander ethnicity, malignancy, and a history of previous VTE. Higher incidence of symptomatic VTE (2% to 3%) were associated with invasive neurosurgery, total hip arthroplasty, major vascular surgery, and radical cystectomy (140). Forty percent to 50% of VTE incidents occurred after discharge from the hospital (140,141), which supports the need for longer anticoagulant prophylaxis after surgery (142).
patients revealed an overall frequency of VTE within 3 months after surgery of 0.8%. Increased risk of VTE was associated with age, Latino and Asian/Pacific Islander ethnicity, malignancy, and a history of previous VTE. Higher incidence of symptomatic VTE (2% to 3%) were associated with invasive neurosurgery, total hip arthroplasty, major vascular surgery, and radical cystectomy (140). Forty percent to 50% of VTE incidents occurred after discharge from the hospital (140,141), which supports the need for longer anticoagulant prophylaxis after surgery (142).
Intravenous Catheters
Oral Contraceptives and Hormone Replacement Therapy
Use of oral contraceptives (OC) increases the risk of VTE. The overall risk of VTE is about three times higher than in nonusers (147). The risk of VTE increases with the dose of estrogen and might be higher with third-generation progestins as compared with second-generation progestins (147,148); however, these differences were not found in some studies (149). The risk of VTE in women taking OC increases dramatically (17-fold) in clinical situations that predispose to VTE (150). Hormone replacement therapy also carries two- to fourfold increased risk of VTE and MI (151,152,153). The risk of venous and arterial thrombosis is further increased in patients with primary or secondary thrombophilia (154,155,156).
Lower and upper extremity deep vein thrombosis
DVT is a common and frequently underdiagnosed disorder, characterized by formation of a thrombus within the lumen of the vein. Venous thrombi are composed mainly from erythrocytes and are dark red in appearance. Usually, they originate in the valve pocket of the deep vein and extend to fill the whole vessel lumen. They are loosely attached to the venous endothelium and can be easily fragmented and cause pulmonary embolization.
The deep venous system of the lower extremities is the most common location of DVT, and the deep calf veins are the most common site in the lower extremity (143,157,158).
Lower Extremity Deep Vein Thrombosis
Lower extremity DVT is difficult to diagnose by means of clinical symptoms only. So-called “typical” symptoms are frequently absent or falsely positive; however, a combination of clinical symptoms and risk factors can often lead to proper diagnosis (159). The majority of lower extremity DVT is asymptomatic (1,160,161) and resolve spontaneously. Frequently, an episode of PE is the first sign of DVT.
Classic local clinical symptoms of lower extremity DVT include unilateral increased calf circumference and/or “pitting” edema of the distal part of extremity, calf pain, and Homan sign (calf pain during passive dorsiflexion of the foot), calf and thigh tenderness, skin discoloration (cyanosis), and erythema. Those symptoms are notoriously nonspecific and have low value for clinicians (162). Therefore, multiple clinical score systems are used since combinations of symptoms have better diagnostic value (163,164,165).
Natural History of Lower Extremity Deep Vein Thrombosis
The deep veins of the calf are the most common location of lower extremity DVT (143,158). Without therapy, up to 25% of calf DVT progresses to the popliteal and femoral veins (166). Spontaneous resolution occurs more frequently in DVT that is limited to the calf (143,166). In patients with isolated calf DVT, Meissner found 50% thrombus load reduction in one month and 100% after 1 year (167). Kim, studying patients with venography after knee arthroplasty, documented thrombus resolution in all patients within 6 months (143).
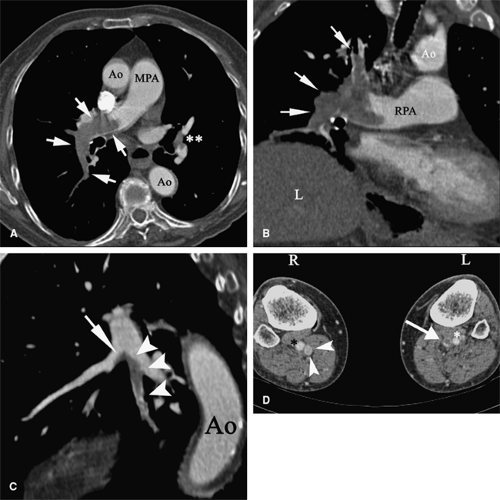
Patients with proximal DVT are more likely to have PE, which occurs in about 50% of patients (166) (Fig. 9-4). Proximal DVT is also more likely to cause chronic venous occlusion (168). Post-thrombotic syndrome (PTS) features chronic pain, edema, hyperpigmentation, and skin ulceration and is a frequent late sequel of DVT. Its incidence varies in different studies from 82% (169) to 11% in patients after hip arthroplasty complicated by DVT (170). Risk factors of PTS after DVT included multilevel DVT, calf DVT, recurrence of ipsilateral DVT, and insufficient oral anticoagulation (169).
Phlegmasia cerulea dolens is an uncommon presentation of severe multilevel lower extremity DVT, usually in patients with malignancy or other causes of thrombophilia. Almost complete obstruction of the venous outflow results in rapidly progressive leg edema with compromise of arterial perfusion and distal gangrene (171). The high mortality rate is the result of hypovolemic shock and preexisting medical conditions (172).
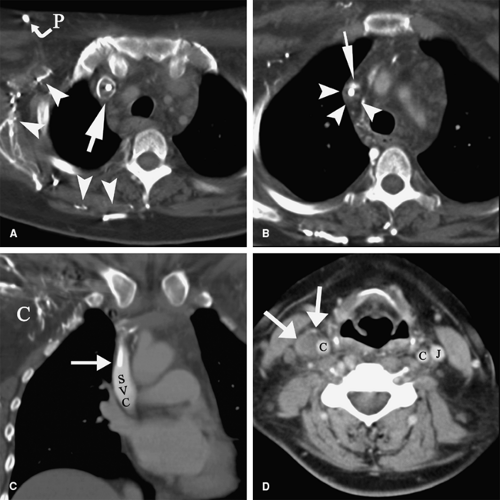
Upper Extremity Deep Vein Thrombosis
DVT in the upper extremities is less common, frequently of iatrogenic origin associated with IV catheters (173) and pacemaker electrodes (174) (Figs. 9-5, 9-6). Other risk factors for upper extremity DVT include malignancy, thrombophilia, previous DVT, IV drug abuse, and thoracic outlet syndromes including effort thrombosis in athletes (175,176,177).
Typical symptoms include upper extremity swelling, skin discoloration, and arm and shoulder pain, although
like lower extremity DVT, upper extremity DVT is frequently asymptomatic (178,179).
like lower extremity DVT, upper extremity DVT is frequently asymptomatic (178,179).
Diagnosis of Extremity Deep Vein Thrombosis
Contrast venography has long been considered the gold standard for the diagnosis of extremity DVT. However, ultrasound examination correlates remarkably well with venographic findings (181) and became widely adopted as the initial screening tool for patients with suspected DVT
in general clinical practice as well as in clinical trials (182). Limitations of ultrasound examination include poor visualization of pelvic vein DVT, isolated calf vein DVT, proximal subclavian vein DVT, and poor visualization of other veins in the presence of significant leg edema. Despite limitations, ultrasound examination can be safely used to make therapeutic clinical decisions in patients with suspected DVT (183,184).
in general clinical practice as well as in clinical trials (182). Limitations of ultrasound examination include poor visualization of pelvic vein DVT, isolated calf vein DVT, proximal subclavian vein DVT, and poor visualization of other veins in the presence of significant leg edema. Despite limitations, ultrasound examination can be safely used to make therapeutic clinical decisions in patients with suspected DVT (183,184).
The differential diagnosis of lower extremity DVT includes muscle cramps, aggravated chronic venous insufficiency, superficial thrombophlebitis, Baker’s cyst, cellulitis, lymphedema, myositis, panniculitis, neuralgia, vasculitis, calf hematoma, arthritis, and leg injury.
Treatment of Extremity Deep Vein Thrombosis
The cornerstone of extremity DVT therapy is anticoagulation. Initial therapy includes IV heparin or low-molecular-weight heparin (LMWH) administered subcutaneously (SC) or IV. A significant advantage of LMWH is the ability to carry on therapy on an outpatient basis, resulting in significant cost savings.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree