Contrast Medium Administration in Computed Tomographic Angiography
Dominik Fleischmann
Intravenous (IV) contrast medium (CM) administration is one of the essential ingredients of computed tomographic angiography (CTA). Its primary goal is to achieve adequate opacification of the vascular territory of interest synchronized with the CT acquisition. This seemingly simple goal is not always easy to achieve, notably in the light of continuously evolving CT technology, where scan times have become substantially shorter with each new scanner generation. Empirically developed injection protocols designed for the scanning times achievable in the early days of CTA are no longer adequate for current multiple detector-row computed tomography (MDCT) technology. Fast acquisitions require that physiologic constraints of arterial enhancement have to be taken into account when building an integrated scanning and injection protocol for a cardiovascular CT application. The key to any rational design of contrast medium injection protocols is a thorough understanding of the physiologic and pharmacokinetic principles governing arterial enhancement, and knowledge of the effects of user-selectable injection parameters on vascular enhancement.
This chapter first reviews the general properties and safety aspects of iodinated contrast media with particular
relevance to CT angiography. The central purpose is, however, to explain the basic principles of arterial enhancement (early contrast medium dynamics) for CT angiography, which are timeless and thus independent of the scanner generation used. Finally, this chapter reviews how the basic principles and currently available techniques of contrast medium delivery can be assembled into rational injection protocols for state-of-the-art cardiovascular CT.
relevance to CT angiography. The central purpose is, however, to explain the basic principles of arterial enhancement (early contrast medium dynamics) for CT angiography, which are timeless and thus independent of the scanner generation used. Finally, this chapter reviews how the basic principles and currently available techniques of contrast medium delivery can be assembled into rational injection protocols for state-of-the-art cardiovascular CT.
Contrast Media for Computed Tomographic Angiography
Angiographic x-ray CM currently in use are water-soluble derivates of symmetrically iodinated benzene (tri-iodobenzene). They are either negatively charged ionic (ionic CM) or nonionic (nonionic CM) monomers or dimers, respectively (Table 4-1). In addition to ionicity and chemical structure (monomer vs. dimer), CM are also categorized into osmotic classes (high-osmolar, low-osmolar, and iso-osmolar). The diagnostic use of x-ray contrast media is exclusively based on the physical ability of iodine to absorb x-rays, and not on pharmacological effects, which are generally undesired. The desired effect of CM is thus directly related to the iodine concentration of the agent. The increase in CT numbers observed in a given vessel or tissue after contrast medium administration is directly proportional to the local iodine concentration (1). Other important physicochemical properties of iodinated contrast agents in the context of CT are their osmolality and their viscosity.
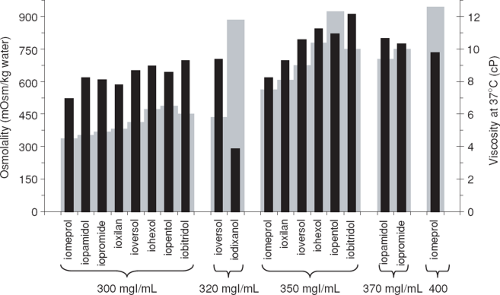
Viscosity has been considered a less important factor than osmolality when mechanical power injectors were first introduced (2); however, in the setting of CT angiography where increasing iodine administration rates are desired, viscosity has regained importance. Furthermore, viscosity may also play a role in CM-induced nephropathy (3), among many other possible factors. The viscosity of a contrast agent depends mainly on the nature of the active ingredient, iodine concentration, and temperature. Viscosity increases exponentially with increasing iodine concentration. Viscosity is reduced by approximately 50% when warmed from room temperature (20°C) to body temperature (37°C) (4). There are distinct differences between nonionic CM of the same iodine concentration with respect to their viscosity (Fig. 4-1). Dimers are the largest molecules and are generally more viscous than monomers.
The selection of IV contrast medium for CTA is primarily governed by safety considerations and the rate of expected adverse reactions. In this context, nonionic CM are generally safer than ionic contrast media, with less idiosyncratic (nondose-dependent, e.g., allergylike) adverse reactions (5,6,7,8). Ionic CM also have a greater potential to cause acute nausea and vomiting, and—as a result—motion, when injection rates greater than 2.0 to 2.5 mL/s are used. Furthermore, extravasation of ionic CM is less well tolerated than nonionic CM. Therefore, nonionic CM are preferable in the setting of CTA (9) and are used almost exclusively today.
Safety Issues of Contrast Media for Computed Tomographic Angiography
While modern nonionic iodinated contrast agents are among the safest medications used worldwide (10), adverse effects occur in approximately 3.13%. An overview of adverse events relevant to CTA is given in Table 4-2. Most reactions to nonionic agents are mild to moderate, but severe reactions do occur in 0.04% (5). Adverse reactions
are generally categorized into idiosyncratic (nondose dependent), allergylike reactions and nonidiosyncratic (dose dependent) reactions. The risk of idosyncratic adverse reactions to intravenously injected contrast medium can be expected to occur in the setting of CTA at the same rate as in any other indication for IV contrast medium use. Acute allergylike reactions are particularly troublesome because of their unpredictability, and also because they may be life threatening. The equipment and expertise to treat acute adverse reactions should be readily available whenever contrast agents are administered. Because serious reactions are infrequent, it is important to review treatment protocols regularly and to check equipment and drugs at regular intervals for expiration and availability. Guidelines for patient screening, premedication, and recognition and management of acute adverse reactions have been published elsewhere (11,12,13). Delayed cutaneous reactions to contrast agents, presumably immune mediated, may occur 1 to 7 days after administration and are thus rarely recognized by radiologists. However, their incidence is surprisingly high when actively looked for (14). Delayed reactions are clinically important, and they have a tendency to recur with reinjection of the same contrast agent (15), which underscores the importance of accurate documentation of any contrast administration.
are generally categorized into idiosyncratic (nondose dependent), allergylike reactions and nonidiosyncratic (dose dependent) reactions. The risk of idosyncratic adverse reactions to intravenously injected contrast medium can be expected to occur in the setting of CTA at the same rate as in any other indication for IV contrast medium use. Acute allergylike reactions are particularly troublesome because of their unpredictability, and also because they may be life threatening. The equipment and expertise to treat acute adverse reactions should be readily available whenever contrast agents are administered. Because serious reactions are infrequent, it is important to review treatment protocols regularly and to check equipment and drugs at regular intervals for expiration and availability. Guidelines for patient screening, premedication, and recognition and management of acute adverse reactions have been published elsewhere (11,12,13). Delayed cutaneous reactions to contrast agents, presumably immune mediated, may occur 1 to 7 days after administration and are thus rarely recognized by radiologists. However, their incidence is surprisingly high when actively looked for (14). Delayed reactions are clinically important, and they have a tendency to recur with reinjection of the same contrast agent (15), which underscores the importance of accurate documentation of any contrast administration.
Table 4-1 Physicochemical Properties of Radiographic Contrast Media | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Table 4-2 Adverse Events of Contrast Media, Risk Factors, and Preventive Measures in the Setting of Computed Tomographic Angiography | ||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||
Manifest hyperthyroidism is considered an absolute contraindication for iodinated contrast media. Contrast medium–induced thyrotoxicosis is otherwise rare. Patients at risk of developing thyrotoxicosis after contrast medium injection are patients with Graves disease as well as patients with multinodular goiter with thyroid autonomy, especially elderly patients and patients living in areas of iodine deficiency (the United States is not an iodine deficiency area). Routine monitoring of thyroid function tests before contrast medium injection in patients with a normal thyroid is not indicated (16). Patients at high risk should be carefully monitored by endocrinologists after contrast medium examinations. The free iodide load of contrast media interferes with iodide uptake in the thyroid. Contrast media therefore compromise diagnostic thyroid scintigraphy and radio-iodine treatment of thyroid malignancies for 2 months after administration and should be avoided in this setting. Simple guidelines on the CM and thyroid function have recently been published (16).
Because CM delivery for CTA requires comparably large volumes injected at high flow rates with power injectors, it is important not to ignore the potential risks of dose-dependent adverse effects of CM. Based on clinical and experimental evidence for ionic CM, one might naturally assume that rapid injections would be less well tolerated than slower injections (17). However, at least for injection flow rates up to 4 mL/s, no correlation between injection rate and the overall rate of adverse reactions has been found (18). Utilization of noninvasive cardiovascular imaging is increasing (19), however, and there is an increased use of CT particularly in the elderly (20). This patient population is more likely to have cardiovascular disorders and diabetes, and may be more vulnerable than other current and past patient cohorts undergoing CT.
The “classic” dose-dependent adverse reactions in the ionic CM era (particularly with intra-arterial or intracoronary injections) included nausea, vomiting, arrhythmia, pulmonary edema, and cardiovascular collapse. The dose-dependent effects that require specific attention in the setting of CTA are CM extravasation, cardiovascular effects, and CM-induced nephrotoxicity (CIN). Drug interactions are also a concern in this patient population.
Contrast Medium Extravasation
Extravasation is a well-known complication of IV CM administration. The rate of extravasation has been reported to range from 0.2% to 0.6% when mechanical power injectors are used (21). Although injection flow rates of 5 mL/s for CTA were reported as early as 1993, many radiologists were reluctant to inject more than 3 mL/s. Over the years, however, injection flow rates for CTA have increased, and flow rates of 5 or 6 mL/s have become clinically routine at many institutions today. Although there has been concern that these injection flow rates would be associated with a proportionally increased risk of extravasation, no correlation between extravasation frequency and injection rate has been found (18). Ten mL/s are standard for functional imaging studies using CT (22,23). In the era of IV digital subtraction arteriography (DSA), the standard peripheral IV injection rate was 15 mL/s (24) for volumes of 30 to 50 mL, repeated for each view.
Most extravasations of CM involve only small volumes and result in minimal to mild symptoms if nonionic CM is used. When large volumes of CM extravasate, it is usually in noncommunicative patients such as infants and children, the elderly, or unconscious patients. Severe extravasation injuries such as skin necrosis and ulceration, or compartment syndrome, have occasionally been reported even with nonionic agents (21). Thus, a preliminary rapid manual injection of saline with the patient’s arm in scanning position (e.g., above the head) is generally recommended to ensure correct and stable cannula position prior to iodinated CM administration. Monitoring of the injection site early during CM administration reduces the risk of significant extravasation but does not eliminate it because extravasation may occur
several seconds after the beginning of the injection. The use of a CM extravasation detection device connected to the power injector reduces this risk as well (25).
several seconds after the beginning of the injection. The use of a CM extravasation detection device connected to the power injector reduces this risk as well (25).
Conservative management of CM extravasation injury is often adequate, but it is advisable to establish a local policy for management of these injuries together with a plastic or hand surgeon (21,26). Both warm and cold compresses may be beneficial to the treatment of local extravasation. There is no clear consensus on the preferred treatment. Proper documentation of extravasation events is important.
Cardiovascular Effects
Cardiovascular adverse reactions to intra-arterial (notably coronary) but also to intravenous injections of ionic CM are well documented experimentally and clinically, and include arrhythmia, tachycardia, pulmonary edema, and cardiovascular collapse (17,27). With IV administration of nonionic agents, serious cardiovascular adverse events are probably very rare.
Cochran et al. analyzed the trends of adverse events to more than 90,000 CM injections over a period of 15 years (from 1985 to 1999) at their institution (28). As expected, they found decreasing rates of adverse events when the universal use of ionic CM was replaced with selective use of nonionic CM followed by universal use of nonionic contrast agents. Interestingly, the authors found that seven of the ten severe reactions to nonionic CM occurred in patients after CT angiography. The authors also found that severe reactions with nonionic contrast agents were more likely due to cardiopulmonary decompensation than to allergylike reactions in their patients. While the authors could not exclude a statistical glitch and a change in the patient population during the study period (which is not at all unlikely), a potential association between cardiopulmonary decompensation and CT angiography is not excluded.
Because severe reactions to nonionic agents are rare in general, and because it is probably not always easy to differentiate allergylike from cardiopulmonary events retrospectively (and both may coexist), one cannot expect to easily find hard evidence to prove or exclude an increased risk of CTA to cause cardiopulmonary adverse events. For patients with substantial cardiopulmonary compromise, however, it is quite plausible that a rapidly injected volume of CM can result in acute decompensation.
Theoretically, the risk can be reduced when smaller volumes and flow rates are used. In patients with low cardiac output, this will still result in good vascular opacification. Unfortunately, it is unlikely that patients at risk will be easily identifiable beforehand. Individualizing the injection flow rates and volumes to body size is at least one rational technique to avoid excessive CM injections in older persons with small stature.
Contrast Medium–Induced Nephrotoxicity
CIN is commonly defined as an impairment of renal function with an increase of serum creatinine of >25% or 0.5 mg/dL (44 μmol/L) from baseline within three days after CM injection in the absence of alternative etiology (29). The mechanism is incompletely understood and may be due to a combination of several factors, such as a direct toxic effect on renal tubular cells (30), and contrast medium–related changes in renal medullary perfusion subsequent hypoxemia (3). Osmolality and viscosity may both play a role, but at this point, it is quite controversial how and to which extent these factors contribute to CIN (3,31). CIN due to nonionic contrast agents is rare in the general population (less than 2%), but the incidence may be greater than 25% in patients with risk factors (32). The primary risk factors are pre-existing renal impairment, notably if associated with diabetes, volume depletion, and the use of nephrotoxic drugs. While CIN is reversible in the vast majority of cases, it may cause considerable morbidity and mortality. Patients who develop CIN are at significantly higher risk of death, both in the hospital and at 1 year, at least in patients undergoing cardiac catheterization and angiography (33).
There are two practical consequences in the setting of CTA. It is necessary to screen for patients at risk, and once a patient at risk is identified, it is necessary to take measures to reduce the likelihood of CIN. Detailed discussions of this topic and specific guidelines have again been published elsewhere (13,34).
Screening for Patients at Risk
A protocol to identify patients at risk of CIN should be in place in every diagnostic imaging department. The practice of screening these patients in the setting of CTA depends mostly on the clinical setting (outpatients vs. inpatients or emergency room). In an outpatient setting, routine measurement of serum creatinine is not necessary in patients younger than 70 years if a simple questionnaire designed to elicit a history of renal disorders and additional risk factors for CIN suggests normal renal function (35). If the questionnaire indicates a positive history of prior kidney disease, diabetes, kidney surgery (including transplantation), or congestive heart failure (Table 4-2), the serum creatinine should be measured. Recently, a rapid strip-based test has become commercially available that allows bedside determination of serum creatinine and BUN within a few minutes (36), which may be particularly helpful in the outpatient setting. In inpatients, serum creatinine levels should always be known before CM administration.
Table 4-3 Serum Creatinine Levels (in mg/dL) Indicating an Estimated Glomerular Filtration Rate of less than 60 mL/min/1.73 m2 | ||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||
Serum creatinine is an imperfect marker of renal dysfunction, and in the setting of CIN, its primary limitation is that a fixed threshold of “normal” creatinine (e.g., 1.3 mg/dL) may not detect even a more than 50% reduction of the glomerular filtration rate and thus miss a significant proportion of patients at risk. It has been suggested that a patient’s glomerular filtration rate should be estimated instead of using serum creatinine. This can be done most easily using the 4-variables abbreviated version of the original 6-variable MDRD (modification of diet in renal disease) formula (37,38,39), which calculates a patient’s glomerular filtration rate per body surface area (thus correcting for body size already), based on patient age, sex, race, and serum creatinine. When an estimated glomerular filtration rate equal to or less than 60 mL/min/1.73 m2 is used as a threshold to identify patients at risk, one can again simplify the matter and tabulate the creatinine thresholds above which a patient would be considered to have renal insufficiency and thus be at risk for CIN (Table 4-3).
Prevention in Patients at Risk
In patients at high risk for CIN, other imaging modalities such as magnetic resonance imaging (MRI) without contrast medium and Doppler ultrasound should be considered first. If CTA is deemed necessary, the lowest possible dose of a low osmolar or iso-osmolar dose should be used (Table 4-4). With fast scanners, and if only large vessel disease is clinically suspected such as for aortic aneurysm follow-up and evaluation, small volumes and flow rates may suffice, but one should not risk that image quality is nondiagnostic, particularly if acute treatment decisions need to be inferred from a CTA.
Nephrotoxic drugs, such as nonsteroidal anti-inflammatory drugs (NSAID), should be stopped at least 24 hours before CM administration. Volume expansion with IV fluid is known to reduce the risk of CIN. It is not entirely clear if oral hydration would be adequate as well; it certainly does no harm. Studies have shown that it is important to start fluid injection (e.g., 100 mL per hour of normal 0.9% saline) several hours before CM injection and to continue for several hours thereafter (40). A recently published more compact hydration protocol by Merten et al. (41) using bicarbonate seems to be effective and in the setting of CTA has the advantage that it can be started only 1 hour before contrast medium injection (Table 4-4).
There are numerous studies, meta-analysis, and reviews published regarding the pharmacological prevention of CIN using vasodilators (e.g., fenoldopam), receptor antagonists of endogenous vasoactive mediators (e.g., theophyllin), or cytoprotective drugs (e.g., acetylcysteine), all of which do not seem to offer consistent protection against CIN (40). Despite conflicting evidence in the literature, acetylcysteine may still be useful in a pretreatment protocol (together with volume expansion with bicarbonide) in patients with increased risk of CIN because of its low cost, the lack of side effects, and simplicity of use (Solomon R, personal communication).
While it is well recognized that low-osmolar nonionic contrast agents are less nephrotoxic than high-osmolar ionic contrast agents in patients with renal impairment, it is currently unclear if and to what extent iso-osmolar contrast agents (42) further reduce the risk of CIN compared with low-osmolar agents, notably in the setting of IV injections for CTA. More study is required to confirm the reported lower nephrotoxicity of iso-osmolar contrast media. Finally, if patients at risk for CIN undergo a CT angiographic study, it is also important to ensure that serum creatinine levels are being followed-up adequately.
Drug Interactions
Contrast media may interact with the pharmacological action of other drugs. Given the increasing use of CM and
the changing patient population, including older patients and patients with multiple medical problems (20), it is important to be aware of these interactions. Knowing a patient’s drug history and proper documentation of the CM used are the first of several general guidelines on how to avoid drug interactions with CM (43). In the setting of CTA, the following drugs need special attention.
the changing patient population, including older patients and patients with multiple medical problems (20), it is important to be aware of these interactions. Knowing a patient’s drug history and proper documentation of the CM used are the first of several general guidelines on how to avoid drug interactions with CM (43). In the setting of CTA, the following drugs need special attention.
Table 4-4 Practical Preventive Measures for Contrast Medium–induced Nephrotoxicity in the Setting of Computed Tomographic Angiography | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||
Metformin may cause lactic acidosis in diabetic patients with renal impairment, notably in diabetic nephropathy, and should be discontinued (44).
Nephrotoxic drugs such as cyclosporine, cisplatin, aminoglycosides, and NSAIDs and also loop diuretics enhance the adverse effects of CM on renal function. A few drugs may enhance allergylike reactions to CM. The most important ones in the context of cardiovascular CT/CTA are beta-blockers. Patients on beta-blockers are significantly more likely to have an anaphylactoid reaction than matched controls (45,46). Furthermore, beta-blockers reduce the effectiveness of adrenaline when a life-threatening anaphylaxislike reaction occurs (11). Contrast media may not only precipitate interleukin-2 (IL-2) toxicity, but patients on IL-2 also have higher rates of immediate urticarial as well as delayed reactions to IV CM. An increased risk may remain for 2 years after the end of treatment. Previous CM reaction in an IL-2 patient should be considered a relative contraindication of further CM (47). Finally, it has been suggested to avoid contrast medium injections in patients treated with hydralazine




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree