Thoracic Imaging in the Critically Ill
Imaging of the thorax in critically ill and/or intensive care unit (ICU) patients has an important role in successful patient management. Portable chest radiography is the mainstay for evaluation of the thorax in these patients. Chest radiographs are either performed as daily routine examinations or are performed as urgent or immediate examinations due to abrupt changes in clinical status or the manipulation of indwelling diagnostic, therapeutic or monitoring lines, tubes, and devices. The latter are covered in detail in Chapter 11. Performing daily routine portable chest radiographs on ICU patients has been shown to detect unexpected problems, with subsequent alterations in the diagnostic approach or treatment after nearly 40% of “routine” examinations and after nearly two-thirds of all portable chest radiographs on ICU patients (1,2,3). Although routine testing faces considerable scrutiny by health care facilities and third-party payers facing constant pressure to reduce costs, such imaging for critically ill intensive care unit patients may be justified as an effective method to discover unexpected problems that may be a source of morbidity or mortality. Guidelines for portable radiography can be developed successfully by multidisciplinary teams, so that imaging is appropriate (4,5).
Daily routine portable radiographs in ICU patients commonly reveals significant findings.
Important elements of acute care radiology include not only the timely performance and accurate interpretation of examinations, but prompt communication of abnormalities to the ordering physician. Daily radiology rounds between radiologists and the ICU team, including physicians, nurses, and respiratory therapists, promotes quality patient care. Bitetti and Zimmerman (6) described the concepts of such a team, indicating the positive benefits of improved diagnostic information, facilitated sequencing of studies, and decreased hospital length of stay.
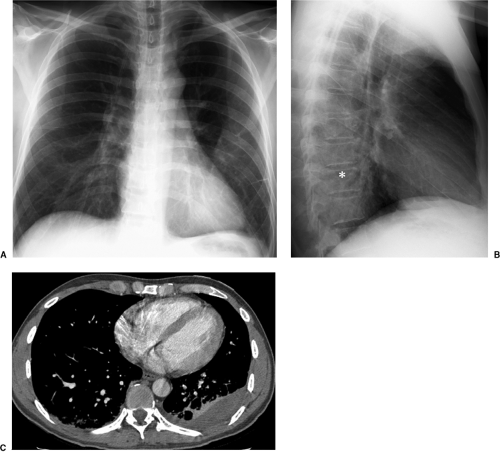
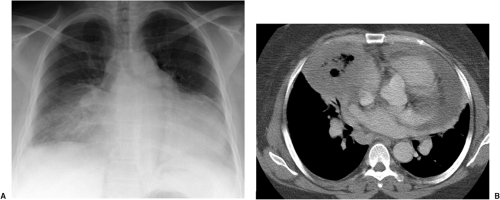
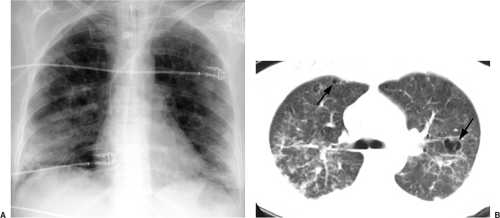
In the last decade, computed tomography (CT) has played a greater role in the imaging of critically ill ICU patients (7,8,9,10,11). Clinical indications for CT in this setting include distinguishing between pleural and parenchymal disease (Fig. 10.1) and evaluating pleural fluid collections, including empyema (Chapter 17), lung abscess (Fig. 10.2), mediastinal abnormality, abnormal or unusual fluid or air collections (Fig. 10.3), and pulmonary embolism (Chapter 21). CT may also be used for CT-guided percutaneous drainage and fluid aspiration (12). CT for ICU patients generally requires the transportation of ill and often medically unstable patients to the radiology department, with careful attention to the logistical difficulties of maintaining life support systems during transportation. Physiologic changes are common during transportation, as frequently occurs when these patients
are in the ICU. In one study, 39% of all patients transported to radiology had a change in management within 48 hours of the diagnostic examination; for abdominal CT examinations, that number was even higher, at 51% (13). At our institution the transportation of adult ICU patients to radiology is done by a specialized team of six registered nurses with critical care training, called the SWAT team (Smiling, Willing, Able and Technical), a concept developed at the University of Missouri (14). Up to two SWAT nurses and a respiratory therapist usually transport the ICU patients, depending on their clinical status. Use of specially trained nursing staff has been shown to reduce adverse outcomes associated with intrahospital transportation of critically ill patients (14).
are in the ICU. In one study, 39% of all patients transported to radiology had a change in management within 48 hours of the diagnostic examination; for abdominal CT examinations, that number was even higher, at 51% (13). At our institution the transportation of adult ICU patients to radiology is done by a specialized team of six registered nurses with critical care training, called the SWAT team (Smiling, Willing, Able and Technical), a concept developed at the University of Missouri (14). Up to two SWAT nurses and a respiratory therapist usually transport the ICU patients, depending on their clinical status. Use of specially trained nursing staff has been shown to reduce adverse outcomes associated with intrahospital transportation of critically ill patients (14).
Mobile or portable CT scanners are also available to use in or adjacent to the ICU (15,16). In general, image quality is poorer than the modern fast helical CT scanners that are prevalent today. Portable CT scanners are not technically capable of performing fast CT angiographic examinations, as required to evaluate for suspected pulmonary embolism or vascular emergencies. They are capable of basic evaluation of the lungs, pleural spaces and mediastinum, abdomen, and intracranial structures. In one series, the physicians who ordered portable CTs cited that patient severity of illness, use of extracorporeal life support, and cardiovascular instability were the most common indications for the study; when faced with a situation in which the portable CT scanner was not available, 67% of physicians
ordered fixed helical CTs requiring transportation to the radiology department (15). In contrast to CT, ultrasound examinations may be performed at the bedside and are particularly useful for the evaluation of pleural fluid collections, both for diagnosis and percutaneous ultrasound-guided intervention (Chapter 22) (17,18).
ordered fixed helical CTs requiring transportation to the radiology department (15). In contrast to CT, ultrasound examinations may be performed at the bedside and are particularly useful for the evaluation of pleural fluid collections, both for diagnosis and percutaneous ultrasound-guided intervention (Chapter 22) (17,18).
Radiologic Approach
Comprehensive and systematic assessment of chest radiographs in critically ill patients includes evaluation of lung parenchymal abnormalities, pleural disease, mediastinum, cardiovascular structures, and physiologic parameters. The visualized portion of the abdomen should also be evaluated for signs of disease processes that are often silent clinically in intubated, sedated, and/or paralyzed patients. It is important to look at the musculoskeletal structures as well. The correct position of all diagnostic, therapeutic, and
monitoring devices should be confirmed, as detailed to a greater extent in Chapter 11. The use of computed radiography with soft-copy interpretation of images on workstations by radiologists improves the delivery of portable chest images and facilitates the initiation of clinical actions, without any reduction in diagnostic quality, when compared with conventional film-screen radiography (19,20). As a by-product, the use of computed radiography also reduces consultation with radiologists.
monitoring devices should be confirmed, as detailed to a greater extent in Chapter 11. The use of computed radiography with soft-copy interpretation of images on workstations by radiologists improves the delivery of portable chest images and facilitates the initiation of clinical actions, without any reduction in diagnostic quality, when compared with conventional film-screen radiography (19,20). As a by-product, the use of computed radiography also reduces consultation with radiologists.
Table 10.1: Lung Parenchymal Opacification in the ICU Setting | ||
|---|---|---|
|
The interpretation of portable chest radiography is made more difficult by the limited power output of portable equipment and subsequent inconsistency of filming technique. The latter can be overcome in many cases by the use of computed radiography, which allows manipulation of image parameters at a computer workstation to optimize evaluation of the thorax. Patient factors also contribute to reduced image quality and include the inability to position patients fully upright, the inability to obtain images free of respiratory motion at full inspiration in intubated and often medically paralyzed patients, and the superimposition of the internal and external components of lines, tubes, and devices.
Causes of lung parenchymal abnormality are discussed here, including acute respiratory distress syndrome (ARDS), acute interstitial pneumonitis (AIP), infection (Chapters 5 and 6), atelectasis, aspiration, and both cardiogenic and noncardiogenic pulmonary edema (Table 10.1). Abnormal air collections are then discussed, including pneumomediastinum, pneumopericardium, pneumatoceles, and interstitial emphysema. Pneumothorax is discussed briefly here in the setting of barotrauma and in greater detail in Chapters 12 and 17.
Pulmonary Parenchymal Opacification
Acute Respiratory Distress Syndrome
Definition
Previously termed adult respiratory distress syndrome, the modern definition of acute respiratory distress syndrome (ARDS) was reported in 1967 by Ashbaugh et al. (21). Today, ARDS is defined as the onset of acute respiratory failure, accompanied by severe and persistent hypoxemia despite the administration of high concentrations of inspired oxygen (ratio of arterial partial pressure of oxygen-to-fraction of inspired oxygen less than 200 mm Hg), pulmonary capillary wedge pressure (PCWP) less than 18 mm Hg, and the absence of elevated left heart filling pressures, with consolidation in three or four quadrants of the lungs on the chest radiograph. This definition was reported by a joint American–European Consensus Conference on ARDS (22). The definition had previously included diffuse pulmonary opacity, shunt physiology, increased dead space, and decreased lung compliance in the absence of increased left-sided filling pressures (23). Systemic inflammatory response syndrome may be a precursor to or trigger acute lung injury, ARDS, and multiple organ system failure (24). Systemic inflammatory response syndrome is defined as a systemic inflammatory response to a variety of clinical insults and is manifested by two or more of the items
listed in Table 10.2 (24). There are many potential proinflammatory and antiinflammatory mediators of acute lung injury, and it is the balance between these factors that culminates in the clinical manifestations of acute lung injury and ARDS. The former includes platelets and white blood cells, cytokines, endorphins and histamine, endotoxins, and vasoactive neuropeptides. The latter includes interleukins, epinephrine, leukotriene B4 receptor antagonist, and lipopolysaccharide binding protein.
listed in Table 10.2 (24). There are many potential proinflammatory and antiinflammatory mediators of acute lung injury, and it is the balance between these factors that culminates in the clinical manifestations of acute lung injury and ARDS. The former includes platelets and white blood cells, cytokines, endorphins and histamine, endotoxins, and vasoactive neuropeptides. The latter includes interleukins, epinephrine, leukotriene B4 receptor antagonist, and lipopolysaccharide binding protein.
Table 10.2: Systemic Inflammatory Response Syndrome (Defined as Two or More of the Items Below) | ||
|---|---|---|
|
Clinical Presentation and Risk Factors
ARDS is a clinical syndrome and is diagnosed clinically not radiographically. Patients with ARDS usually present acutely with rapidly progressive dyspnea, tachypnea, and respiratory distress. The risk factors for ARDS are listed in Table 10.3, with sepsis accounting for up to 35% of cases (25). Risk factors are synergistic, with more risk factors further increasing the risk of ARDS. The mortality of ARDS remains high, between 40% and 60%, despite advances in treatment and understanding of the pathophysiology of ARDS. There has been a gradual reduction in mortality. In general, mortality from ARDS is lower in patients less than 60 years of age and in patients with ARDS secondary to sepsis (26).
ARDS is a clinical syndrome, not a radiographic diagnosis.
Radiography and Phases
Although the diagnosis of ARDS is based primarily on clinical findings, the chest radiograph may provide additional diagnostic information concerning the effectiveness of treatment, complications, and prognosis. Bachofen and Weibel (27) described the three pathologic phases of ARDS lung injury and response in 1977: the acute exudative phase/stage, a fibroproliferative phase, and a fibrotic phase or healing stage. The acute phase, represented pathologically by diffuse endothelial cell injury with alveolar capillary leak of proteinaceous fluid and neutrophils, manifests radiologically as diffuse ill-defined alveolar opacities predominantly in the lung periphery (Fig. 10.4A) (28). As capillary leak
progresses, with greater extravasation of fluid from the intravascular space into the alveoli, widespread pulmonary opacification and complete “white-out” of the lungs occurs radiographically (Fig. 10.4B) (29). Grossly, the lungs are heavy and wet. Other microscopic pathologic features include platelet microthrombi and white blood cells in the capillary lumen, swelling of capillary endothelial cells, infiltration by polymorphonuclear leukocytes, and hyaline membrane formation within the alveoli. Injury to alveolar epithelial
cells results in decreased surfactant production and therefore decreased lung compliance, reflected on radiographs as small lung volume and atelectasis (27).
progresses, with greater extravasation of fluid from the intravascular space into the alveoli, widespread pulmonary opacification and complete “white-out” of the lungs occurs radiographically (Fig. 10.4B) (29). Grossly, the lungs are heavy and wet. Other microscopic pathologic features include platelet microthrombi and white blood cells in the capillary lumen, swelling of capillary endothelial cells, infiltration by polymorphonuclear leukocytes, and hyaline membrane formation within the alveoli. Injury to alveolar epithelial
cells results in decreased surfactant production and therefore decreased lung compliance, reflected on radiographs as small lung volume and atelectasis (27).
Table 10.3: Risk Factors for Acute Respiratory Distress Syndrome | |
|---|---|
|
Table 10.4: Radiologic Manifestations of Barotrauma | |
|---|---|
|
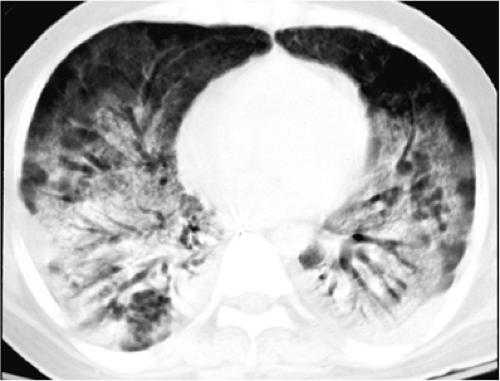
In general, during the acute exudative phase, the alveolar opacities of ARDS progress over several days on chest radiographs, until there is diffuse or near diffuse opacification of the lungs. Subsequently, the appearance changes very slowly from day to day. The alveolar edema of ARDS is not associated with widening of the vascular pedicle, cardiomegaly, or altered pulmonary blood flow distribution, which is in contrast to cardiogenic, uremic, and hypervolemic pulmonary edema. Because capillary leak occurs directly into the alveolar spaces, septal lines are usually absent on chest radiographs in ARDS. Although the pulmonary vessels are generally not visible through the alveolar opacities, when seen they may be vasoconstricted. In the subacute phase, which occurs over the next 5 to 10 days after the acute phase, the pathologic findings are proliferation of epithelial cells and fibroblasts, together with collagen deposition. This produces the radiographic findings of progressive lung destruction and a transition from alveolar to combined alveolar and interstitial opacities (Fig. 10.5). Findings of barotrauma (Table 10.4), including pneumothorax and pneumatocele formation, are frequent during this fibroproliferative phase, during which fibrosis has developed and the lungs become stiff and noncompliant (30,31). Although some patients recover from ARDS with no pulmonary function deficit, other patients eventually enter the chronic phase several weeks after the initial lung injury, manifested by fibrosis and focal emphysema on chest radiographs.
ARDS generally changes slowly on radiographs from day to day.
Early recognition of barotrauma in ARDS patients is important to minimize potentially serious complications, such as tension pneumothorax.
Computed Tomography
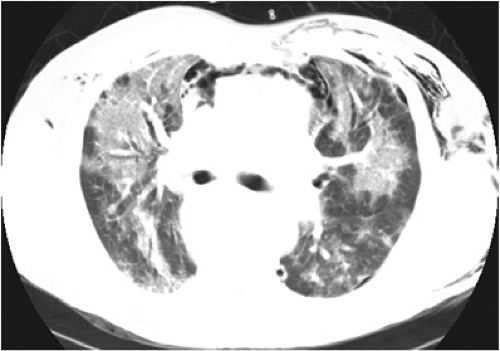
CT of ARDS was first reported in the early-to-mid 1980s and has been studied extensively since that time. Much of the work has been performed by Gattinoni and colleagues (32,33,34). What was once thought to be a disease process that homogeneously involved both lungs based on chest radiography is now recognized to be a more heterogeneous process based on CT investigations. The CT appearance varies with the etiology of ARDS, mechanical ventilation, patient position, and time. For example, in ARDS secondary to direct lung injury, such as pneumonia or aspiration, the radiologic appearance is more patchy and multifocal. In contrast, in ARDS with indirect lung injury, such as sepsis or sustained hypotension, lung injury is more diffuse (Fig. 10.6) (35). CT is clinically useful in patients with ARDS who are not improving or are deteriorating clinically, by identifying pleural effusions, lung abscess, lobar atelectasis, barotrauma, and malpositioned lines and tubes more accurately than chest radiographs (36,37).
Ground glass opacity, consolidation, and a reticular pattern are the hallmarks of ARDS on CT. The ground glass opacity is believed to represent active inflammation in lung interstitium and alveolar wall, coupled with incomplete alveolar filling by inflammatory cells, cellular debris, and edema. Consolidation is due to either complete filling of the alveolar spaces with fluid and debris, and/or atelectasis, referred to as the collapse of potentially recruitable lung units. If a patient were to be placed prone, the potentially recruitable lung units may reexpand and fill with air, thereby improving gas exchange. This has lead to the theory that patients with ARDS should be rotated regularly, with changes in the distribution of lung abnormality demonstrated on CT with changes in position (34,38). The reticular pattern may be seen both acutely, secondary to edema or inflammation, or in the chronic phase of ARDS, representing fibrosis. During the acute phase of ARDS, the anterior
or most nondependent lung may be normal or near normal, whereas the mid-third of the lungs in the ventral to dorsal direction is ground glass in attenuation, with consolidation in the dorsal or most dependent lung, creating a ventral to dorsal gradient of lung attenuation. A cranial-to-caudal gradient is also noted, being more consolidated in the lower lungs than at the apices.
or most nondependent lung may be normal or near normal, whereas the mid-third of the lungs in the ventral to dorsal direction is ground glass in attenuation, with consolidation in the dorsal or most dependent lung, creating a ventral to dorsal gradient of lung attenuation. A cranial-to-caudal gradient is also noted, being more consolidated in the lower lungs than at the apices.
If ARDS resolves within 1 week, there are usually few if any radiologic sequelae. With a more protracted course of ARDS, the exudated fluid is reabsorbed from the lung and the fibroproliferative organizing phase sets in. Lung attenuation improves, whereas signs of fibrosis, including distortion of the normal bronchovascular architecture, may be found. Subpleural cysts, also known as pneumatoceles, varying in size from a few millimeters to a few centimeters, develop in both the dependent and nondependent lung.
Follow-up high resolution CT of the lungs after ARDS demonstrates fibrosis in most patients (39,40). The extent of fibrosis correlates with the severity of ARDS, the duration of mechanical ventilation using high peak inspiratory pressures, and a higher fraction of inspired oxygen. The location of the fibrosis is predominantly in the ventral or nondependent lung in the supine position, suggesting that it may be secondary to the high peak inspiratory pressures and oxygen therapy used to treat ARDS rather than to the ARDS itself. The collapsed dependent lung may be spared from this injury, protected by the failure
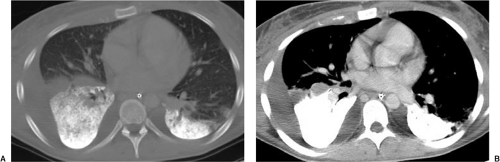
to ventilate this region of the lung. Partial liquid ventilation is an experimental treatment for ARDS, in which perfluorocarbon is instilled into the airway with continuous gas ventilation through the endotracheal tube. Perfluorocarbon is a clear odorless liquid that is radiopaque (41). It distributes within the lungs in a gravity-dependent distribution, which matches the distribution of the most severely consolidated lung in ARDS, where it reduces alveolar surface tension and keeps the alveoli distended to facilitate gas exchange (Fig. 10.7).
to ventilate this region of the lung. Partial liquid ventilation is an experimental treatment for ARDS, in which perfluorocarbon is instilled into the airway with continuous gas ventilation through the endotracheal tube. Perfluorocarbon is a clear odorless liquid that is radiopaque (41). It distributes within the lungs in a gravity-dependent distribution, which matches the distribution of the most severely consolidated lung in ARDS, where it reduces alveolar surface tension and keeps the alveoli distended to facilitate gas exchange (Fig. 10.7).
Acute Interstitial Pneumonitis
AIP is an idiopathic form of acute lung injury, characterized by rapidly progressive cough and dyspnea leading to severe hypoxemia and respiratory failure over days. This short duration is in contrast to other chronic idiopathic interstitial pneumonias, such as usual and nonspecific interstitial pneumonitis that have a more insidious disease onset (Chapter 14). AIP was formerly referred to as Hamman-Rich syndrome. In 1935 Hamman and Rich (42) described five patients with acute lower respiratory tract illness, four of whom died after hospital stays of up to 6 months; one died within days. At autopsy, organizing diffuse alveolar damage and diffuse interstitial fibrosis was present in all cases (42). In 1986 Katzenstein and colleagues (43) coined the phrase “acute interstitial pneumonia” to reflect the acuity of the disease and distinguish it from other chronic interstitial pneumonias. Others later reviewed the autopsy material of the original 1935 cases and compared them with current cases of AIP, confirming that the pathologic lesion of AIP was identical to that described by Hamman and Rich (44).
AIP is acute diffuse alveolar damage of unknown etiology.
AIP should be suspected in patients diagnosed initially with severe diffuse bilateral community-acquired pneumonia that does not respond to broad-spectrum antibiotics and from whom no infectious organism is isolated (45). It is distinguished from ARDS by the absence of a known cause of ARDS and the lack of systemic involvement or multisystem organ failure. Surgical lung biopsy in AIP demonstrates organizing diffuse alveolar damage, the same pathologic lesion seen in the fibroproliferative phase of ARDS (45). Similar to ARDS, the chest radiographic manifestations of AIP are bilateral, patchy, alveolar opacities that may progress to extensive diffuse consolidation. Reported survival in AIP ranges from 26% to 67%; however, the higher numbers likely reflect case series contaminated by ARDS and the lower figure is from a series of well-documented biopsy-proven cases. The number of pathologically confirmed AIP cases is small, and the impact of treatment, such as corticosteroids or cytotoxic drugs that are used for other forms of interstitial pneumonitis, is unclear.
Infection
Pulmonary infections are covered extensively in Chapters 5 and 6. Aspects of infection as they relate to ICU patients are discussed here. The radiographic hallmark of pneumonia is airspace consolidation with air bronchograms, which may be segmental, lobar, or diffuse in distribution. New or progressing consolidation, together with two or more of the following, should raise suspicion for pneumonia: fever, hypothermia, peripheral leukocytosis or leukopenia, purulent respiratory secretions, and worsening respiratory failure (22). In critically ill patients the diagnosis of pneumonia may be delayed or unrecognized, because fever and leukocytosis may be absent. Fever may also be present in at least 50% of patients with atelectasis and no pneumonia, making it a poor clinical sign of infection (46,47). Pneumonia is difficult to diagnose in the setting of ARDS, with a high false-negative rate, as high as 29% (27). In a series by Mock et al. (48), the clinical variables and radiographic findings of 80 patients with positive sputum cultures were reviewed. One point each was given for the presence of new airspace “shadows,” air bronchograms, segmental infiltrates, asymmetric infiltrates, infiltrates in nondependent lung, ipsilateral pleural effusion, and for the absence of volume loss, cardiomegaly, and hilar enlargement on chest radiographs. Clinical symptoms of fever, leukocytosis, respiratory failure, and
mortality did not correlate with the radiographic findings. Patients with high radiographic scores, ranging from 4 to 10, were more likely to have positive blood and fluid cultures, polymicrobial cultures, multisystem organ failure, Escherichia coli or Pseudomonas infection, and improvement on antibiotics.
mortality did not correlate with the radiographic findings. Patients with high radiographic scores, ranging from 4 to 10, were more likely to have positive blood and fluid cultures, polymicrobial cultures, multisystem organ failure, Escherichia coli or Pseudomonas infection, and improvement on antibiotics.
Clinical signs of pneumonia are often absent in critically ill patients.
Table 10.5: Risk Factors for Nosocomial Pneumonia in the Intensive Care Unit | |
|---|---|
|
Nosocomial infections in the ICU setting are of particular concern, both in surgical and medical ICUs. They occur in 20% to 40% of patients, increasing morbidity, mortality, and costs (49,50,51,52). For example, in one series of trauma ICU patients, mortality was 43.5% in the patients with nosocomial pneumonia compared with 18% in the patients without nosocomial pneumonia (49). Risk factors for nosocomial infection are listed in Table 10.5. Prolonged mechanical ventilation is a major risk factor. Endotracheal tubes bypass natural host defenses, allow leakage of bacteria and secretions around the cuff into the airway, damage the ciliated tracheal epithelium, reduce bacterial clearance from the trachea, and direct bacteria into the lungs through tube manipulation and airway suctioning. It has been estimated that for each day of mechanical ventilation, the risk of nosocomial pneumonia increases by 1% (53). The organisms responsible for the infection and the rate of infection may vary from hospital to hospital and even from ICU to ICU within the same hospital (54). CT may be useful in ICU patients with sepsis of unknown origin, identifying the source of fever within the thorax or abdomen in nearly 20% of patients (55).
The risk of nosocomial pneumonia increases by 1% for each day intubated.
Another form of thoracic infection that should be considered in ICU patients is septic pulmonary emboli. ICU patients are at risk due to indwelling vascular catheters for combined underlying infection or nosocomial infection. When patchy bilateral lung parenchymal opacities or wedge-shaped nodularity develops, often subpleural in distribution, the possibility of septic pulmonary emboli should be raised (Figs. 21.24 and 21.25). Although the lung abnormality usually increases slowly, in some cases progression can be quite rapid. The nodules are usually less than 3 cm in size and may cavitate (56). CT is more sensitive than plain radiography for diagnosing septic emboli and may yield a diagnosis before it is clinically or radiographically suspected (56,57,58)
Patchy subpleural alveolar or nodular opacities should raise the suspicion of septic pulmonary emboli.
Aspiration and Aspiration Pneumonia
Critically ill patients are at increased risk of aspiration and aspiration pneumonia. Predisposing reasons for this are given in Table 10.6. Aspiration should be suspected when chest radiographs demonstrate the sudden appearance of new focal alveolar opacities (Fig. 10.8).
Unless occurring acutely related to manipulation of airway or esophageal tubes, episodes of aspiration are often unrecognized by caretakers. Aspiration can be divided into three categories, according to the type of fluid aspirated and subsequent lung reaction: toxic, bland, and infectious (Table 10.7) (59).
Unless occurring acutely related to manipulation of airway or esophageal tubes, episodes of aspiration are often unrecognized by caretakers. Aspiration can be divided into three categories, according to the type of fluid aspirated and subsequent lung reaction: toxic, bland, and infectious (Table 10.7) (59).
Table 10.6: Factors that Contribute to Aspiration in Intensive Care Unit Patients | |
|---|---|
|
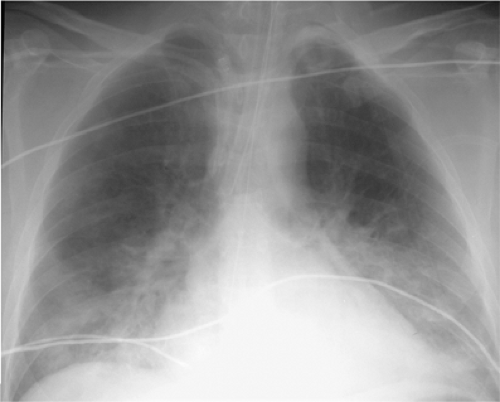 Figure 10.8 Aspiration in an intubated intensive care unit patient manifesting as new bibasilar consolidation. The chest radiograph was normal the previous day and returned to normal 5 days later. |
Acute focal or multifocal alveolar opacities, particularly at the lung bases or in superior segments of lower lobes, should raise the suspicion of aspiration, which is often clinically silent.
Toxic aspiration occurs with the aspiration of acidic gastric contents with an acidic pH of less than 2.5 or water-soluble radiographic contrast material. In toxic aspiration, severe bronchospasm and chemical pneumonitis may develop within minutes of aspiration, manifesting radiographically as noncardiogenic pulmonary edema. When massive, toxic aspiration may result in immediate apnea, hypotension, and shock. Half of these patients will subsequently develop fever and leukocytosis, in the absence of infection, confounding the caretakers into diagnosing pneumonia. In milder forms, there may be mild bronchiolitis. The radiographs may also mimic pneumonia; however, unlike pneumonia, the alveolar opacities gradually improve over 1 to 2 days, which is faster than bacterial pneumonia.
When bland fluid, such as water, blood, or fluid, is aspirated, the radiograph may be normal unless a large volume of fluid is aspirated. Transient respiratory distress usually improves after airway suctioning, and there is usually no significant inflammatory lung response. If either toxic or bland fluid is aspirated in conjunction with solid foreign material such as food, the radiographs will demonstrate airway obstruction with distal atelectasis and frequently subsequent pneumonia.
The aspiration of infected material, such as pharyngeal or airway secretions colonized by multiple organisms, results in radiographic findings of pneumonia, with persistent alveolar consolidation. The location of the abnormality is related to patient position during the aspiration event. When supine, the superior segments of the lower lobes, particularly on the right, are most common, followed by the posterior segments of the upper lobes (60). When upright, the basilar segments of the lower lobes are usually involved (Fig. 5.20). If the patient is prone, as is common when using a rotational bed, the abnormality may be located in the anterior segments of the upper or lower lobes, the middle lobe, or lingula. Aspiration occurring when in a decubitus position may involve multiple or even all segments of the dependent lung and completely spare the nondependent lung. Aspiration
of infected contents in hospitalized patients or patients with poor oral hygiene may cause necrotizing pneumonia due to anaerobic organisms, often accompanied by cavitation and empyema. The most commonly implicated organism is Pseudomonas aeruginosa.
of infected contents in hospitalized patients or patients with poor oral hygiene may cause necrotizing pneumonia due to anaerobic organisms, often accompanied by cavitation and empyema. The most commonly implicated organism is Pseudomonas aeruginosa.
The location of opacities due to aspiration depends on patient position during the aspiration event.
Table 10.7: Categories of Aspiration | |
|---|---|
|
Atelectasis
In contrast to the hazy increased lung parenchymal opacity of pulmonary edema, diffuse atelectasis can often be recognized by secondary signs of volume loss, including low lung volumes and crowding of the bronchovascular structures and ribs. In intubated patients, chest radiographic exposure should be timed to peak inspiration of the ventilatory cycle after tidal volume has been delivered, because images at end-expiration will suffer from diffuse atelectasis and therefore be suboptimal for the evaluation of lung disease. Focal bibasilar subsegmental atelectasis is more common and often transient. After cardiac surgery, the left lower lobe is a common location for both atelectasis and pneumonia, secondary to stretching and cold-induced injury of the phrenic nerve (61). Angulation of the x-ray beam is important. With as little as 10 degrees of lordotic angulation the beam is no longer tangential to the apex of the hemidiaphragm, creating pseudo-opacity in the left retrocardiac region that may be interpreted as atelectasis or consolidation behind the heart (62).
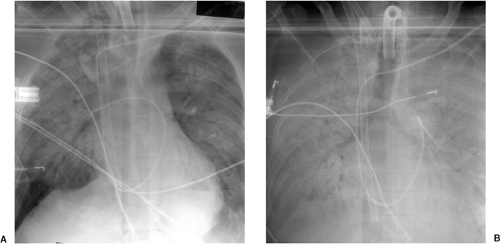
Intubated patients are at increased risk of mucous plugging of the airway due to decreased ciliary function, depressed cough reflex, and increased secretions. Mucous plugging may be a source of acute respiratory decompensation. Whenever acute lobar collapse or even whole lung collapse is identified radiographically, mucous plugging should be suspected. Suctioning of the airway usually results in radiographic and clinical improvement (Fig. 10.9). Atelectasis without air bronchograms is more responsive to suctioning than atelectasis with air bronchograms because of the presence of occlusive secretions within the airway that are amenable to removal (63).
Acute partial or complete lung collapse in an intubated patient is most commonly due to a mucous plug in the airway.
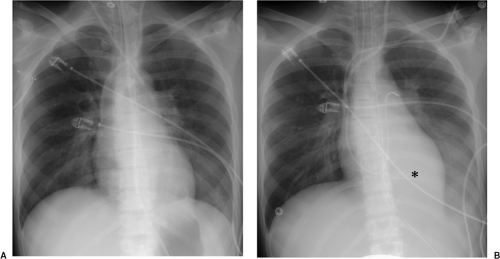 Figure 10.9 Acute lobar collapse secondary to a mucous plug in an intensive care unit patient. A. Initial radiograph demonstrates normal lungs. B. Radiograph a few hours later demonstrates acute left lower lobe collapse with retrocardiac and left lower lung opacity (asterisk) obscuring the left hemidiaphragm, slight shift of the heart toward the left, crowding together of the left posterior ribs compared with the right side, and leftward positioning of the esophagus compared with A as demonstrated by the esophageal tube.
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|