Lung Cancer
Lung cancer is the most common fatal malignancy in humans. It is strongly associated with cigarette smoking and asbestos exposure; viral infections, pulmonary fibrosis, and radiation exposure play a less defined and/or less frequent role (1,2,3,4,5,6,7). Imaging plays a crucial role in detection, diagnosis, and staging.
Detection
Although history and physical examination may suggest a pulmonary process, they seldom result in a specific diagnosis of carcinoma of the lung. Sputum cytology is somewhat better in that it may reveal malignant cells. However, radiology alone allows direct noninvasive visualization of the pulmonary parenchyma and mediastinum.
The chest x-ray (CXR) is the cheapest, easiest, most convenient method for this examination. On the CXR, lesions are often detected when they are in the 1-cm size range, and smaller lesions may be detected. Unfortunately, smaller lesions are most likely to be seen if they are calcified, in which case they are probably benign. Frontal and lateral radiographs increase the likelihood of detecting important small lesions, and comparison with prior radiographs can also improve diagnostic accuracy and assessment of the lesion’s malignant potential. A nodule that has been present for more than 2 years or less than 2 months is unlikely to be malignant, depending on its size and growth rate.
The CXR is the cheapest, easiest, most convenient method for detecting lung cancer.
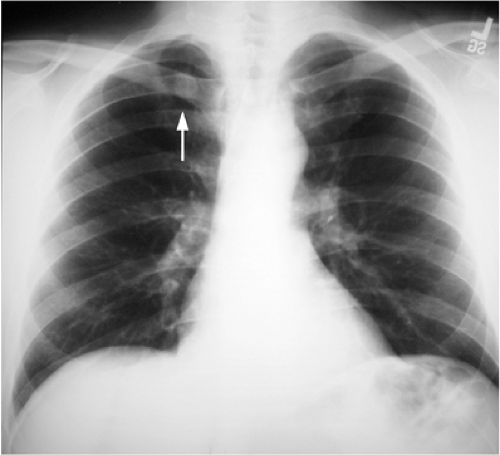
Interpretation of the CXR requires great care and awareness of locations that are more difficult to evaluate (and therefore require more scrutiny). Lesions that may be missed may occur in the lung apices, where overlapping ribs, clavicles, neck soft tissues, and variable pleural and parenchymal scarring from old granulomatous infections result in significant camouflage (Fig. 7.1). “Pseudo-lesions” also occur in this location, because calcification and/or ossification at the first costochondral junctions may simulate lung nodules. Apical lordotic chest radiography, with the patient leaning back and the x-ray beam angled upward, projects anterior structures cephalad, allowing better visualization of the lung apices.
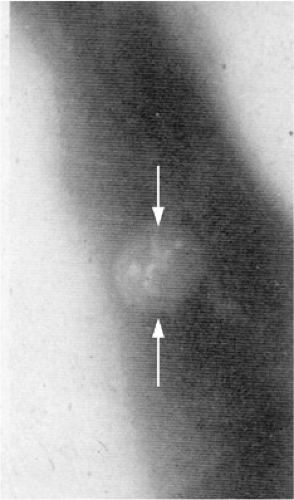
The hila, with branching arteries and superimposed veins, may conceal an enlarged lymph node or obscure a nearby lung nodule. Hilar masses are sometimes better appreciated on the lateral radiograph (Fig. 7.2). The mediastinum may conceal an especially large mass, and lung lesions close to the mediastinum are also harder to detect because of overlap with the heart, aorta and its branches, and superior vena cava. Nodules in any part of the lung may be rendered invisible by surrounding parenchymal abnormality (Fig. 7.3). In
addition, small nodules anywhere at the periphery of the lungs may be overlooked. Shallow oblique radiographs may be a very helpful first step in evaluating a suspicious opacity.
addition, small nodules anywhere at the periphery of the lungs may be overlooked. Shallow oblique radiographs may be a very helpful first step in evaluating a suspicious opacity.
A particularly dangerous nodule location is in the posterior costophrenic sulci of the lungs, below the domes of the diaphragm (Fig. 7.4). In some patients a central endobronchial lesion will clearly be undetectable based on size alone, but careful assessment of secondary signs (such as postobstructive atelectasis, pneumonia, or air trapping) may still allow diagnosis (Fig. 7.5).
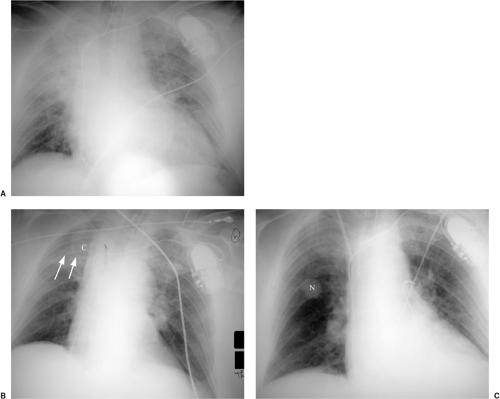
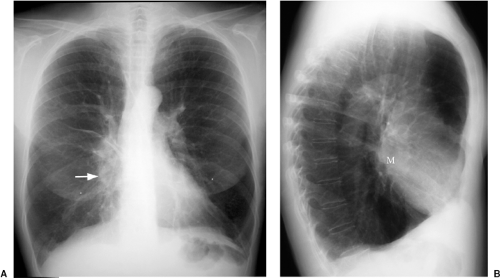 Figure 7.2 Hilar mass better seen on the lateral radiograph. A. Posteroanterior chest x-ray: slight enlargement of right lower hilum (arrow). B. Lateral chest x-ray: mass (M) more easily appreciated. |
To broach the topic of malpractice, a discussion of missed lung cancer (as the flip side of lung cancer detection) is in order. Malpractice has well-defined legal elements (such as duty, breach of duty, negligence, and injury), but jurors are not legal scholars. Jurors may sympathize with young attractive plaintiffs who have obviously suffered from illness; the suffering may not be related to the radiologist in any way. The radiologist is particularly at risk because the images that reveal the radiologist’s “mistake” (important note: mistake does not equal malpractice) are generally available for review, and abnormalities seem to grow before your very eyes in retrospect.
I offer the following ideas for defense of alleged radiologic malpractice in missed lung cancer:
Small lesions: It could not be diagnosed prospectively (usually easy to get supportive expert witness testimony, although it is also easy for the plaintiff to hire someone to testify that they could see this lesion).
Larger lesions: It did not affect the outcome (especially worth consideration with bad cell types like small cell and large cell carcinoma).
The “Mayo Clinic” defense: In a study (8) of CXR screening for lung cancer at 4-month intervals, two or three experts in pulmonary disease (at least one a radiologist)
reviewed radiographs specifically for evidence of lung cancer in high-risk patients (older males who were heavy smokers). When cancer was detected, in 45 of 50 peripheral lung cancers and in 30 of 42 central lung cancers the lesion could also be seen in retrospect on at least one earlier CXR; four were visible on radiographs dating back at least 2 years. Their conclusion: “Our results suggest that failure to detect a small pulmonary nodule on a single CXR should not constitute negligence or be the basis for malpractice litigation.”
The “Where’s Waldo?” defense: In an elegant letter to the editor of the journal Radiology (9), Dr. Ronald Hendrix pointed out the need for an analogy to explain to members of a lay audience how a well-qualified careful radiologist could ever miss a lesion that they now easily see on a radiograph. He likened this to the search for Waldo in the series of “Where’s Waldo?” books. As Dr. Hendrix pointed out, everyone understands how hard it can be to locate Waldo in a given illustration. However, once he has been found, Waldo is incredibly obvious when the same illustration is reviewed. Dr. Hendrix added that it is even harder to look for lung cancer (or any other radiographic finding) because, whereas Waldo is definitely present on every page of a “Where’s Waldo” book, a radiograph may be normal (in other words, it may contain no radiographic findings or Waldos).
I offer the following advice to potential radiologists: If you cannot stand to make mistakes (or more to the point, if you do not want proof of your errors to be part of the permanent medical record), choose another specialty.
Compared with the CXR, computed tomography (CT) is more sensitive for identifying lung nodules; unfortunately, this is accompanied by decreased specificity. Many small nodules detected only at CT are scars, intrapulmonary lymph nodes, or other nonspecific benign lesions. Unfortunately, some are early lung cancers. This is the crux of the issue currently being addressed by trials of CT for lung cancer screening.
Cancer screening would certainly seem to make sense for the leading cause of cancer-related deaths in the United States. However, the biological characteristics of a neoplasm
influence the utility of screening for that neoplasm in an at-risk population. Screening evaluates individuals who manifest no signs of disease; in lung cancer, the at-risk population consists of heavy smokers. For effective screening, the following must apply:
influence the utility of screening for that neoplasm in an at-risk population. Screening evaluates individuals who manifest no signs of disease; in lung cancer, the at-risk population consists of heavy smokers. For effective screening, the following must apply:
Disease must be identifiable before symptoms develop.
Earlier treatment of the disease must be shown to have more effect than later treatment.
Benefits to the few treated for disease must outweigh the expense and harm to the screened population caused by the screening process.
To be considered effective, screening needs to demonstrate a decrease in the mortality of a given disease.
Certain biases need to be considered when evaluating the effectiveness of screening. Lead time bias refers to the fact that earlier detection of disease in a screened population compared with a control group makes it seem that patients live longer in the screened group, even if they die at the same age as control group patients with the same disease. Length time bias means that slowly growing tumors have a longer detectable preclinical phase than rapidly growing tumors and are therefore more likely to be identified at screening. Overdiagnosis bias is the failure to correct for preclinical disease that would not have produced signs or symptoms before the subject would have died of other causes. In general, a large number of subjects (more than 500) and a long observation time (usually more than 5 years) are required to validate the utility of screening.
Four large randomized trials (10,11,12,13) of lung cancer screening in the past 30 years (before the advent of CT screening) failed to detect a statistically significant decrease in lung cancer mortality. This subject is currently being reassessed with the addition of chest CT. CT screening for lung cancer certainly allows identification of disease before symptoms are present. However, it is not yet certain that this can be done cost-effectively, particularly because so many detected nodules turn out to be benign (14,15). In fact, the possibility that screening can even prove harmful has also been raised (16). It is probably best to describe lung cancer screening as a technique that is unproven but not yet discredited.
Lung cancer screening is currently best described as unproven but not yet discredited.
Diagnosis
Lung cancer often manifests as a single nodule or mass. Diagnosis or exclusion of lung cancer starts with an assessment of the radiographic features of the detected nodule or mass. A lesion is generally considered benign if it remains stable for at least 2 years. Thus, the single best test of a lesion’s malignant potential is comparison with old films. This simple fact is so often ignored that it must need more emphasis. Apart from its greater diagnostic accuracy, comparison with old films also results in lower expense (subject to the next rate increase from the U.S. Postal Service) and much less radiation than alternative imaging strategies for assessing a demonstrated lung nodule.
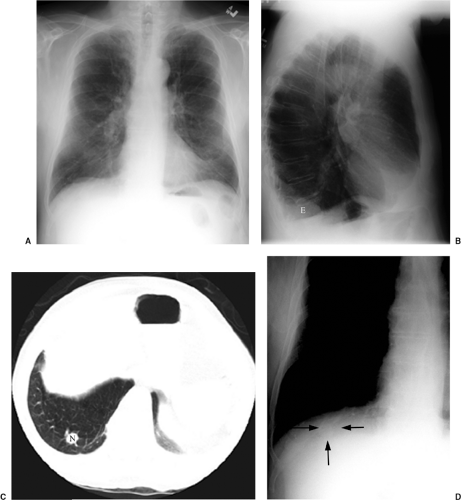
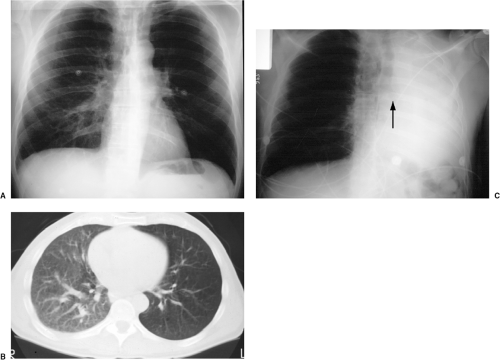
When old films are unavailable, the lesion can be assessed for benign radiographic features. Calcification may be helpful if there is a benign pattern of calcification (diffuse, central, “popcorn,” or concentric) (Fig. 7.6); other patterns of calcification are unrevealing as to a lesion’s malignant potential (Fig. 7.7). Another suggestive finding of benign disease is “rabbit ears” (a feeding artery and draining vein) in a pulmonary arteriovenous malformation (Fig. 7.8). Thin-section CT may be more helpful in this setting, because it may demonstrate calcification that escapes CXR detection (Fig. 7.9), and it can also reveal fat within a lesion (diagnostic of hamartoma) (Fig. 7.10). There has been some enthusiasm for CT assessment of nodule enhancement, with lung cancers enhancing more than benign nodules. Early CT follow-up of a lesion (sometimes in just a few weeks) has also been applied to this issue.
Most of these approaches (apart from comparison with old films) have been superseded by positron emission tomography (PET). PET is the imaging equivalent of comparison
with old films, in that it allows assessment of the activity of a lesion, not just its morphology (Fig. 7.11). We still generally attempt thin-section CT in the assessment of a nodule, but if there is any remaining doubt, PET is generally the next step. We use it increasingly frequently in the assessment of lung nodules, with three caveats:
with old films, in that it allows assessment of the activity of a lesion, not just its morphology (Fig. 7.11). We still generally attempt thin-section CT in the assessment of a nodule, but if there is any remaining doubt, PET is generally the next step. We use it increasingly frequently in the assessment of lung nodules, with three caveats:
A lesion must be 7 mm or greater in size for accurate PET assessment.
Bronchoalveolar carcinoma may be PET negative.
PET has false positives (inflammatory lesions, a particular problem in some parts of the country with abundant fungi such as histoplasmosis or coccidioidomycosis) and false negatives (well-differentiated carcinomas, particularly adenocarcinoma).
Assessment of a lesion’s activity (via PET or comparison with old films) is the crux of imaging a lung nodule or mass.
An important aspect of lung cancer diagnosis is a consideration of the typical CXR appearances of different cell types. The radiographic presentations are emphasized, along with a discussion of the demographics and other features of the various cell types. This is an approach whose merits have been championed by Dr. Michael McCarthy.
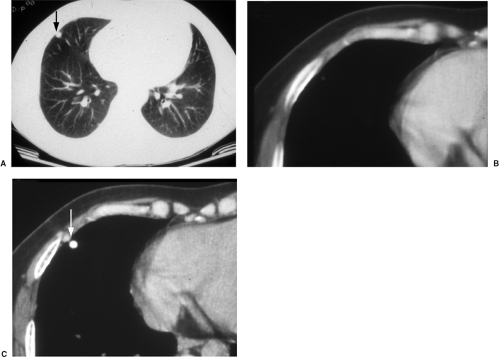 Figure 7.9 Thin-section computed tomography confirmation of nodule calcification. A. Routine computed tomography viewed at lung windows: 1 cm middle lobe nodule (arrow). B. Soft tissue window photography of A: no calcification visible. C. Scan 1 mm thick: nodule is a calcified granuloma (arrow).
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|