Pulmonary Vascular Disease
Pulmonary vascular disease is often overlooked as an etiology for chest pain and dyspnea. Conditions may be life threatening, in the case of acute pulmonary embolism (PE), or may be the cause of chronic symptoms, in the case of pulmonary hypertension and chronic PE. In this chapter we discuss acute and chronic PE, pulmonary arterial hypertension (PAH), and briefly touch on the congenital and acquired abnormalities of the pulmonary arteries, including pulmonary arteriovenous malformations (AVMs), tumor embolism, and stenosis, aneurysms, and tumors of the pulmonary arteries.
Pulmonary Thromboembolic Disease
PE is the third most common acute cardiovascular disease after ischemic heart disease and stroke, and is an important cause of patient morbidity and mortality. The incidence of PE is 600,000 annually in the U.S., and the mortality of untreated PE approximately 30% (1). Hence, it is prudent to diagnose PE accurately and quickly, and to initiate treatment in a timely fashion. Accurate diagnosis is imperative, not only to decrease the morbidity and mortality after treatment, but to avoid the risks of hemorrhage associated with anticoagulation seen in up to 7% of patients, especially in the case of a false-positive test result for PE.
The diagnosis of acute PE continues to pose a challenge to clinicians and radiologists. Ancillary tests include the electrocardiogram, which may demonstrate a right heart strain pattern, and the blood test D-dimer, which when negative is useful to exclude venous thrombosis. However, the D-dimer test is nonspecific and may also be abnormal with myocardial infarction, pneumonia, heart failure, cancer, and recent surgery (2,3).
Venous thrombi form most commonly in veins of the lower extremities and pelvis and then dislodge, propagating cranially into the pulmonary arterial tree. Radiologic evaluation of the patient may include evaluation of the thorax with chest radiography, ventilation-perfusion (V/Q) scans, computed tomography (CT), and magnetic resonance imaging (MRI) and evaluation of the lower extremity veins with CT venography, MR venography, or ultrasound.
Radiology plays a major role in the diagnosis of PE, and the ubiquitous chest radiograph is the first investigation of choice. V/Q scintigraphy has played a major role in the
diagnosis of PE. Though angiography is the standard of reference, it is infrequently used (4,5). The advent of newer helical scanners, electron beam scanners, and particularly multidetector helical scanners has significantly changed the way PE is diagnosed. Multidetector CT is now routinely used in the diagnosis of PE. Magnetic resonance angiography may also be used in the diagnosis of PE and is particularly useful in cases where there is a history of allergy to iodinated contrast media. Transthoracic and transesophageal echocardiography are useful in the diagnosis of large central PE in patients with acute right heart strain and right heart failure. Transesophageal echocardiography can be performed at the bedside and is particularly helpful in medically unstable patients.
diagnosis of PE. Though angiography is the standard of reference, it is infrequently used (4,5). The advent of newer helical scanners, electron beam scanners, and particularly multidetector helical scanners has significantly changed the way PE is diagnosed. Multidetector CT is now routinely used in the diagnosis of PE. Magnetic resonance angiography may also be used in the diagnosis of PE and is particularly useful in cases where there is a history of allergy to iodinated contrast media. Transthoracic and transesophageal echocardiography are useful in the diagnosis of large central PE in patients with acute right heart strain and right heart failure. Transesophageal echocardiography can be performed at the bedside and is particularly helpful in medically unstable patients.
Ultrasound venography is now used as the primary modality for the diagnosis of deep vein thrombosis (DVT) of the lower extremities and has virtually replaced the more invasive direct catheter venography. Magnetic resonance venography is useful, particularly in patients where ultrasound is difficult due to body habitus. Similarly, indirect CT venography is used for evaluating DVT in combination with a CT pulmonary angiogram (CTPA), giving CT the advantage of imaging both the thrombus burden in the extremities and pelvis and the pulmonary arterial tree in a single comprehensive examination. The goal in evaluating suspected venous thromboembolic disease is to have a cost-effective noninvasive test with high sensitivity and specificity.
Etiology and Pathophysiology
PE and DVT are a spectrum of the same disease process, best described as venous thromboembolic disease. PE is a consequence of thrombus, most commonly formed in the deep veins of the pelvis and lower extremities. Thrombus can also originate in the veins of the head, neck, upper extremities, inferior vena cava, right atrium, and right ventricle. Risk factors for DVT are listed in Table 21.1.
In most fatal PE, at autopsy the thrombus is localized proximal to the knee (6). After dislodgement, the venous thrombus propagates through the right heart and into the pulmonary arterial circulation, usually lodging in the main, lobar, or segmental pulmonary arteries, and as a shower of emboli into the subsegmental and smaller pulmonary arteries. In some cases, this leads to acute right ventricular failure. Fatal PE results as a consequence of central occlusive thrombus in patients with normal cardiopulmonary reserve but may also occur with smaller occlusive thrombi in patients with underlying cardiopulmonary compromise. In the uncommon event of a patent foramen ovale, atrial septal defect, or ventricular septal defect, paradoxical emboli may be seen resulting in stroke and mesenteric and renal ischemia.
Pulmonary emboli are more often multiple than solitary and occur more often in the right lung than the left lung. They are most frequently found in the lower lobes, probably due to the greater pulmonary blood flow to the lower lobes. Pulmonary hemorrhage, infarction, or atelectasis may occur as a consequence of PE. Acute PE is treated with either
anticoagulation or, if contraindicated, with an inferior vena cava filter. Treatment with anticoagulants prevents more clot forming while the body dissolves the clot. If this fails, intravenous thrombolysis with recombinant tissue plasminogen activator may be given over a period of 8 to 12 hours and occasionally up to 24 hours in hemodynamically stable patients. In hemodynamically unstable patients, intraarterial thrombolysis as a part of thrombectomy may be performed, with the thrombolytic agent directly injected into the thrombus. Surgical embolectomy may be required in large occlusive PE and carries a high mortality. If thrombolysis fails to resolve the emboli, chronic thromboembolic pulmonary hypertension (CTEPH) may ensue.
anticoagulation or, if contraindicated, with an inferior vena cava filter. Treatment with anticoagulants prevents more clot forming while the body dissolves the clot. If this fails, intravenous thrombolysis with recombinant tissue plasminogen activator may be given over a period of 8 to 12 hours and occasionally up to 24 hours in hemodynamically stable patients. In hemodynamically unstable patients, intraarterial thrombolysis as a part of thrombectomy may be performed, with the thrombolytic agent directly injected into the thrombus. Surgical embolectomy may be required in large occlusive PE and carries a high mortality. If thrombolysis fails to resolve the emboli, chronic thromboembolic pulmonary hypertension (CTEPH) may ensue.
Pulmonary emboli are usually multiple and located in areas of greatest pulmonary blood flow.
Table 21.1: Risk Factors for Pulmonary Thromboembolic Disease | |
|---|---|
|
Clinical Presentation
The clinical manifestations of PE are nonspecific and include dyspnea, chest pain that is often pleuritic in nature, hemoptysis, cough, syncope, or even death. Tachypnea, tachycardia, atrial fibrillation, hypotension, fever, and a pleural friction rub may be noted on physical examination (7). Because of the nonspecific nature of the signs and symptoms, the diagnosis is often overlooked or confused with other diagnoses. Arterial oxygen saturation is often low, especially in cases of significant PE. The electrocardiogram may demonstrate tachycardia, atrial fibrillation, or a right heart strain pattern with S1Q3T3 pattern or a new right bundle branch block; more commonly the electrocardiogram is normal.
Radiology in the Diagnosis of Acute Pulmonary Embolism
Chest Radiograph
Although the chest radiograph is usually the first imaging examination performed in patients with suspected PE, it may be completely normal even in the presence of near fatal PE. When abnormal, the findings are usually nonspecific. Chest radiographic findings are uncommon due to the dual blood supply from bronchial arteries. The main use of a chest radiograph is to exclude other disease processes that mimic the clinical signs and symptoms of PE, such as pneumonia, pneumothorax, rib fractures, aortic dissection, pleural effusions, pericardial effusion, tumor, and hiatal hernia (8). The radiograph is used in conjunction with the V/Q scan in the evaluation of PE, because it is used in the interpretation scheme for V/Q scans (9).
Chest radiographs in patients with suspected PE are most useful to exclude other disease processes that may be causing symptoms, such as pneumonia or edema.
No chest radiograph, normal or abnormal, can be used to exclude PE.
The chest radiograph findings are given in Table 21.2. Historical findings of PE on chest radiography are rarely encountered (Table 21.2), such as the Westermark sign, which is pulmonary oligemia distal to an obstructing embolus (Fig. 21.1), and the Fleischner sign, which is a large central pulmonary artery due to central thrombus with abrupt tapering, also known as the knuckle sign. These signs are mainly seen when there is PE without infarction. The Hampton hump, a wedge-shaped pleural based opacity with the apex pointing toward the hilum and the base abutting the pleura, particularly along the diaphragmatic pleura, is highly suggestive of pulmonary infarction. Pulmonary infarcts maintain their shape as they resolve, which may take several months. Occasionally, an infarct is mistaken for a solitary pulmonary nodule. Although most infarcts resolve completely, many leave an area of linear scar and occasionally a small nodule.
Westermark sign, Fleischner sign, and Hampton hump are classic but very uncommon radiographic findings of PE.
Table 21.2: Chest Radiograph Findings of Acute Pulmonary Embolism | ||||||||
|---|---|---|---|---|---|---|---|---|
|
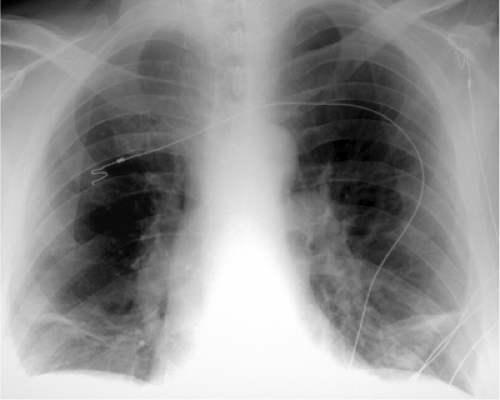 Figure 21.1 Westermark sign. Chest radiograph demonstrates pulmonary oligemia in the right mid-lung, secondary to central right pulmonary embolus. |
The nonspecific radiograph findings in PE with infarction include an elevated hemidiaphragm, pleural effusion, and atelectasis. Pleural effusions are quite common and are often small and unilateral. These often occur early and are frequently hemorrhagic. Occasionally, they can be large or bilateral. Effusions may be an isolated finding in up to one-third of patients with PE and are seen in approximately two-thirds of patients when accompanied by pulmonary hemorrhage or infarction.
Most chest radiographs in pulmonary embolism are nonspecific.
In the original Prospective Investigation of Pulmonary Embolism Diagnosis (PIOPED) study, the sensitivity and specificity of the V/Q lung scan was determined using pulmonary angiogram as the gold standard in the evaluation of suspected acute PE (10). The study concluded that clinical assessment combined with the V/Q scan established or excluded the diagnosis of acute PE in only a small number of patients. A normal chest radiograph was uncommon, found in 12% of patients. Radiographic signs poorly correlate with the diagnosis of PE and did not help to confirm or exclude the diagnosis of PE.
Radionuclide Imaging
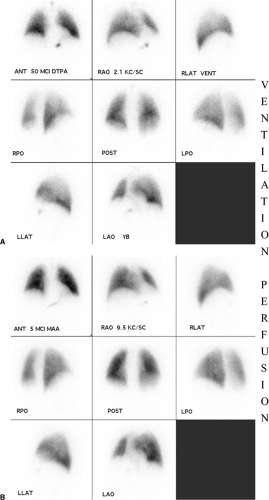
The principle underlying V/Q scintigraphy in the diagnosis of PE is the detection of decreased or absent perfusion, with corresponding normal ventilation. Perfusion scintigraphy is performed by intravenous injection of technetium-99m–labeled albumin macroaggregates ranging from 10 to 100 μm in size. These microparticles lodge in the precapillary arterioles of the pulmonary vascular bed. Eight static views are obtained (anterior, posterior, both laterals, and both anterior and posterior obliques). Ventilation scintigraphy is most often obtained after inhalation of radiolabeled gas such as xenon-133 or aerosols such as technetium-99m diethylenetriaminepentaacetic acid or technetium-99m pyrophosphate. Again, eight images are obtained as above, preferably in the upright position and especially with xenon. A normal V/Q scan is illustrated in Fig. 21.2.
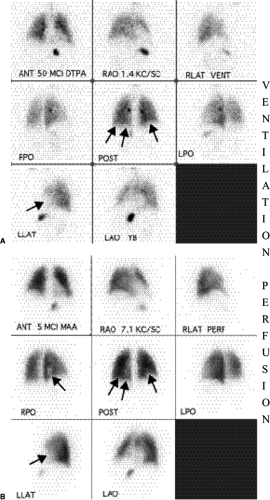
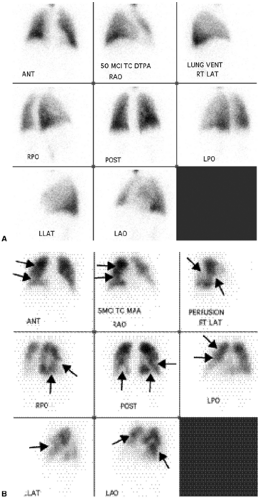
Although the diagnosis of PE is based on a mismatched perfusion defect (i.e., perfusion defect in an area that is normally ventilated), the test is not specific for the diagnosis of PE. Most V/Q scan interpretations require interpretation in conjunction with a current chest radiograph. The current criteria used in the diagnosis of PE are the revised PIOPED criteria (11,12) (Table 21.3). Using these criteria, a V/Q scan is labeled as normal, very low probability, low probability, intermediate/indeterminate probability (Fig. 21.3), and high probability (Fig. 21.4) for the diagnosis of PE. Although a normal V/Q scan virtually excludes a PE and a high probability scan confirms a PE, in the PIOPED study only 14% of patients had a normal V/Q scan and 13% of patients had a high probability V/Q scan. The other 73% of patients had an indeterminate V/Q scan, a nonconclusive result requiring further evaluation with other studies, such as pulmonary angiography.
V/Q scans for PE are most specific when perfusion defects occur in areas of normal ventilation.
Pulmonary Angiography
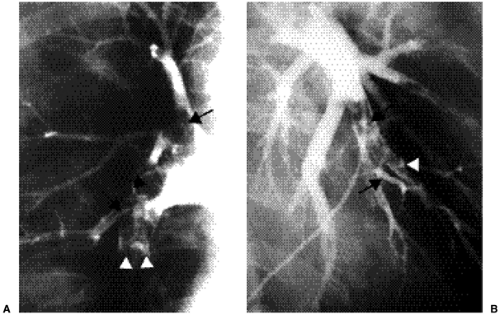
Pulmonary angiography is considered the reference test for the diagnosis of PE, against which all other tests are compared. Pulmonary angiography is recommended when there is an indeterminate V/Q scan, a discordance between the V/Q result (low probability, very low probability), and a high clinical suspicion for PE, indeterminate CTPA, or in cases of acute massive PE. It is particularly useful when catheter-guided thrombolysis or catheter-guided thrombectomy with direct injection of thrombolytic agent into the thrombus is to be performed. An invasive test, it requires a venous puncture usually at the groin and placement of a catheter through the right atrium and right ventricle into the main pulmonary artery, followed by selective placement in the right and left pulmonary arteries. Passage through the heart may induce an arrhythmia, including right bundle branch block. Therefore, patients with left bundle branch block should have a pacemaker in during the procedure. Contrast media is then injected and images obtained in the anteroposterior and oblique projections of each lung. It is a relatively safe invasive test, with a low mortality rate of 0.5% and morbidity rate of 3% to 5%, but is an extremely underused modality, used in only 12% to 14% of patients with nondiagnostic V/Q scans (4,13,14,15). Pulmonary angiography allows direct visualization of the clot burden. The angiographic signs of PE are a filling defect within an opacified pulmonary artery (Fig. 21.5) or occlusion of a pulmonary arterial branch (16,17). Absent or reduced opacification of small arterial branches is sometimes seen, corresponding to a perfusion defect on perfusion scintigraphy. A good quality negative pulmonary angiogram virtually excludes PE. However, angiography has its limitations. It is an invasive test that is underused and not available at all centers.
Table 21.3: Revised PIOPED Ventilation Perfusion Interpretation Categories and Criteria | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||
Pulmonary angiography is the reference test (gold standard) for the diagnosis of PE.
Pulmonary angiography is underused in the diagnosis of PE.
Computed Tomography
PE has incidentally been encountered on thoracic CT examinations as early as 1978. With the advent of new, faster, helical CT scanners, the ability of CT to detect PE as a primary test has received a great deal of interest (18,19). Techniques and scanners have evolved over the last decade; since the introduction of four-row multidetector and greater detector CT scanners, CT has taken on a more dominant role in the evaluation of suspected PE, right down to the subsegmental level (20). CTPA has been almost as reliable as pulmonary angiography in the detection of PE up to the segmental level; with multidetector CT, this reliability may also extend to the subsegmental level (21). Up to one-third of cases at the subsegmental level are misdiagnosed, even at pulmonary angiography (10). With thinner collimation, subsegmental emboli can be seen in 61% to 70% of cases (19,22). There is ongoing debate about the clinical significance of isolated subsegmental PE and whether these should be treated. Treatment may especially benefit patients with cardiopulmonary compromise; in patients with normal cardiopulmonary physiology there is more debate.
Helical Computed Tomography Pulmonary Angiogram
Iodinated contrast, 120 to 150 mL, is usually injected via the antecubital vein, at 4 to 5 mL/s. The timing of intravenous contrast bolus is crucial in obtaining diagnostic quality images. The technique has to be optimized for the CT scanner being used. Scanning is done from caudal to cranial direction to minimize streak artifact from the superior vena cava and to avoid breathing motion artifact from the diaphragm, during the latter part of the scan, if the patient cannot breath-hold.
Using single detector CT, the patient is scanned from the domes of the diaphragm to the top of the aortic arch in a single breath-hold following a scan delay of 25 seconds. Images are obtained at 2.5 mm to 3 mm collimation and reconstructed at 1.5 to 2 mm intervals. Using the 4 row multidetector CT scanner, because of the scanner speed, the entire chest can be scanned in 17 to 22 seconds, following a scan delay of 20 seconds at 1.25 mm collimation with images reconstructed at 0.625 mm intervals. Using 8-row detector multidetector CT the scan time is reduced again by half and on 16-row detector multidetector CT, to one-fourth, or approximately 5 seconds—of great benefit in patients with shortness of breath.
CTPA is interpreted on a workstation and read using a scrolling mode with altering of the window level and width dynamically for optimal evaluation of the pulmonary arteries. The scrolling mode is used to pan through the main, lobar, segmental, and subsegmental pulmonary arteries; hence, it is extremely important to be familiar with the pulmonary arterial anatomy.
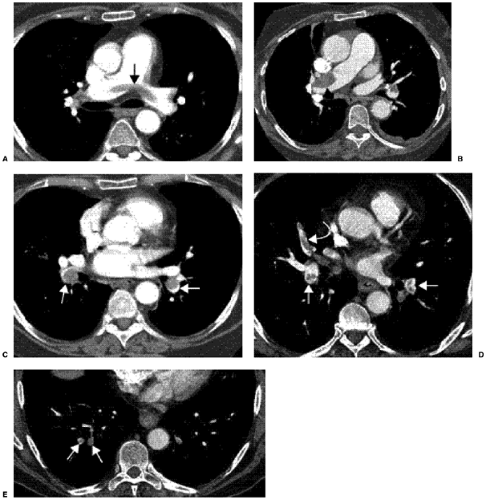
Acute PE is principally diagnosed by visualizing a low attenuation filling defect within a well-opacified pulmonary artery (Figs. 21.6 and 21.7). Other CT findings of acute PE are listed in Table 21.4. Some of these signs include the “railway track” sign (Fig. 21.8D), the vessel cutoff sign, and the rim sign (Fig. 21.8C), where there is a filling defect due to thrombus with a rim of contrast around it. If there is occlusive thrombus, the corresponding artery may be larger and completely filled with low attenuation thrombus. In the case of nonocclusive thrombus, the rim sign may be seen or low attenuation thrombus can be seen in the center of the artery as a nonadherent thrombus or occasionally at the periphery. Ancillary signs of PE, although nonspecific by themselves, can be helpful in case of subtle thrombus. These can manifest as small pleural effusions, atelectasis, or pulmonary infarct distal to a PE (Fig. 21.8), oligemia or mosaic attenuation (Fig. 21.9) due to differential perfusion, and, in case of massive PE, signs of right heart strain with enlargement of the right ventricle and straightening of the interventricular septum.
Intraluminal filling defects on CT are very specific for the diagnosis of PE.
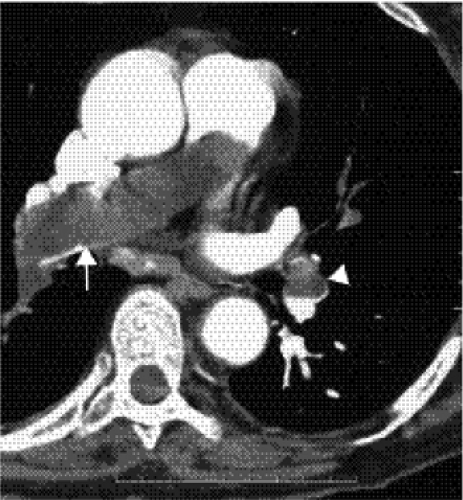
The source of thrombus can occasionally be seen on the chest CT, such as in the superior vena cava, brachiocephalic veins, other neck veins, or the right atrium (Fig. 21.10). Pulmonary infarcts appear as triangular areas with a broad base in contact with the pleural surface and apex directed centrally, with a feeding vessel at the apex. Classic pulmonary infarcts do not enhance, whereas atelectasis or consolidation due to pneumonia often enhances. An advantage of CT is its capability of detecting thoracic abnormalities that mimic signs and symptoms of PE, aiding appropriate and immediate management of these patients (23). These abnormalities manifest as pleural, parenchymal, pericardial, or chest wall disease, seen in up to 40% to 70% of patients with suspected PE, such as pneumonia or abscess (Fig. 21.11), tumor (Fig. 21.12), septic emboli, malignant or nonmalignant pleural effusions, pericardial effusion (Fig. 21.13), pneumothorax, and chest wall tumors.
The neck, extremity, and mediastinal veins should all be examined on pulmonary embolism CTs for the source of emboli.
Pitfalls in the diagnosis of PE on CT are listed in Table 21.5. It is important to use the scrolling mode, so as not to mistake a vein for an artery, an adjacent perihilar node for an arterial filling defect, or mucoid-filled dilated bronchi for an arterial filling defect. Poor contrast bolus causing inadequate opacification of the pulmonary arteries, extensive breathing motion artifact, cardiac motion artifact, low signal-to-noise ratio due to large
patient size, and artifact from tubes and lines in critically ill patients can sometimes pose a challenge in the evaluation of PE in these patients (24,25,26).
patient size, and artifact from tubes and lines in critically ill patients can sometimes pose a challenge in the evaluation of PE in these patients (24,25,26).
Table 21.4: Findings of Acute Pulmonary Embolism on Computed Tomography | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree