The Thoracic Aorta
Many articles and books have been written about the aorta. However, there is still confusion regarding the aorta and aortic diseases among radiologists. Specifically, confusion exists regarding the various terms used to describe acquired aortic diseases, progression of disease, and the postoperative aortic imaging features. In this chapter we discuss the congenital aorta, coarctation, and pseudocoarctation. Acquired aortic diseases are discussed, such as aortic dissection, intramural hematoma, penetrating ulcers, aneurysms, and rare tumors. Finally, the imaging features of the aorta after surgical intervention are described. The goal of this chapter is to clarify the terminology and provide a better understanding of the aorta and the diseases that affect it.
Aortic Dissection
Acute aortic dissection is the most dramatic and common thoracic aortic emergency. Untreated dissections have a mortality of 36% to 72% within the first 48 hours and 62% to 91% within the first week. Dissections are classified as acute if the diagnosis occurs within 2 weeks or less of the first symptoms. Dissections detected after 2 weeks are classified as chronic. Acute aortic dissection usually occurs in patients between the ages of 30 and 85 years, with a peak incidence in the sixth and seventh decades of life. It is more common in men than women, at a ratio of 3:1 (1,2).
There are many conditions that increase a patient’s risk of aortic dissection, as described in Table 18.1. These include systemic hypertension (90%) and connective tissue disorders such as Marfan syndrome, cystic medial necrosis, Ehlers-Danlos syndrome, and Turner syndrome. Aortic dissection noted in women younger than 40 years old is often associated with pregnancy. Congenital cardiovascular diseases, such as aortic stenosis, bicuspid aortic valve, and aortic isthmic coarctation, are all risk factors. Aortic dissection can also be induced by trauma, either blunt or iatrogenic during aortic cannulation or bypass surgery. A history of aortic surgery also increases the risk of subsequent dissection.
Presenting Symptoms
The classic clinical presentation of aortic dissection is the acute onset of severe tearing or ripping substernal chest pain with radiation to the back. This occurs in up to 70% of patients. Aortic valvular murmur due to creation of aortic valve insufficiency occurs in up to 65% of patients. Asymmetric pulses, as well as absent femoral pulses (25%), may occur in the upper extremities if the aortic arch is involved. Unfortunately, 15% to 20% of
patients have no chest pain, making the diagnosis difficult. These patients usually present with symptoms related to secondary involvement of aortic branch vessels. Such presentations include myocardial infarction and congestive heart failure, abdominal pain due to mesenteric ischemia, stroke, confusion, coma, or syncope with involvement of the cranial or spinal arteries (25%) (1).
patients have no chest pain, making the diagnosis difficult. These patients usually present with symptoms related to secondary involvement of aortic branch vessels. Such presentations include myocardial infarction and congestive heart failure, abdominal pain due to mesenteric ischemia, stroke, confusion, coma, or syncope with involvement of the cranial or spinal arteries (25%) (1).
The classic presentation of aortic dissection is acute tearing substernal pain radiating to the back. However, atypical presentations are common and often result in delayed diagnosis.
Table 18.1: Risk Factors for Aortic Dissection | ||
|---|---|---|
|
Pathogenesis
Aortic dissection is a tear of the aortic wall intima, followed by separation of the tunica media, thereby creating two channels for passage of blood. The true lumen is surrounded by intima, and the false lumen is surrounded by media. This is seen in approximately 70% of cases. The tear in the intima allows blood to enter the aortic wall and extend longitudinally along the wall, thus separating the media and forming true and false lumens. The entry point most commonly arises in the ascending aorta within several centimeters of the aortic root or in the descending aorta between the origin of the left subclavian artery and ligamentum arteriosum. Iatrogenically induced aortic dissections arise at sites of aortic cannulation, bypass grafting and cross-clamping, or during catheterization.
All aortic dissections have blood (hematoma) within the media of the aortic wall.
Classification
There are two widely used systems for the classification of aortic dissections: the Stanford classification and the DeBakey classification.
The exact etiology of dissection is still debated. The entry tear may come first, leading to hematoma in the aortic wall, or may arise after the hematoma has already developed. In some cases a tear is never found.
Stanford Classification
Stanford A dissections involve the ascending aorta, regardless of the site of tear or distal extent. These dissections usually begin approximately 2 cm cephalad to the sinotubular junction. These comprise approximately 60% of all aortic dissections. Stanford B dissections involve only the descending aorta distal to the left subclavian artery and comprise approximately 40% of all dissections (3).
Stanford type A dissections involve the ascending aorta. Stanford type B do not.
DeBakey Classification
A DeBakey I dissection involves both the ascending and descending aorta. DeBakey II involves only the ascending aorta, and DeBakey III involves only the descending aorta distal to the left subclavian artery. These occur as 30%, 20%, and 50% of dissections, respectively (4).
DeBakey type I dissections involve ascending and descending thoracic aorta. Type II involves only the ascending aorta and type III, only the descending aorta.
Dissections involving the ascending aorta are treated emergently and surgically because of the high mortality if not treated. Complications if untreated include acute aortic insufficiency in approximately 90% of patients due to destruction of aortic valve, acute heart failure, occlusion of coronary or supraortic branch vessels, rupture into the pleural space, or rupture into the pericardium with acute cardiac tamponade (1). Dissections involving only the descending aorta are associated with a lower complication rate and are treated medically when possible. However, risk of descending thoracic aorta ruptures increases at a greater than 6 cm diameter. This is accompanied by altered branch vessel perfusion or occlusion or pseudocoarctation syndrome with uncontrollable hypertension;
surgical intervention or therapeutic percutaneous stent grafting is required (1,5). Branch vessel occlusions, such as hepatic, mesenteric, or renal artery involvement, are associated with increased morbidity and mortality.
surgical intervention or therapeutic percutaneous stent grafting is required (1,5). Branch vessel occlusions, such as hepatic, mesenteric, or renal artery involvement, are associated with increased morbidity and mortality.
Ascending aortic dissection is a surgical emergency, because rupture into the pericardium, aortic valve failure, and coronary artery involvement are causes of mortality.
Imaging
The methods used for the diagnosis of aortic dissection include catheter aortography (the traditional gold standard) and bedside transesophageal echocardiography. More commonly, noninvasive techniques, such as computed tomography (CT) and magnetic resonance imaging (MRI), are used both to establish the diagnosis of dissection and to evaluate the extent of dissection in the aorta and branch vessels.
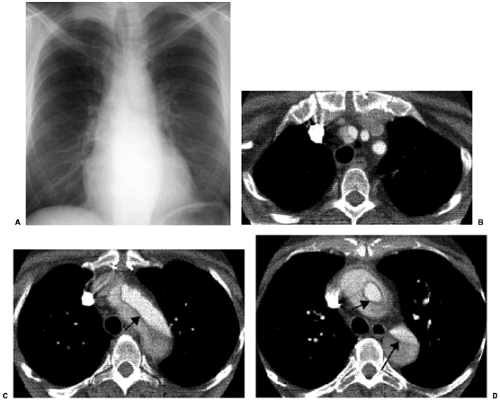
Chest radiographs are the most commonly used radiologic examination in all acute thoracic conditions. Although findings can indicate the presence of aortic dissection (Table 18.2), a normal chest radiograph does not exclude the presence of an aortic dissection (Fig. 18.1).
A normal chest radiograph does not exclude the diagnosis of aortic dissection.
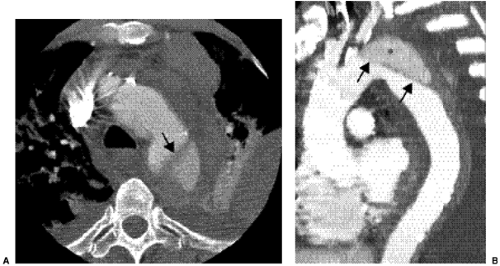
CT is probably the most commonly used radiologic examination to diagnose and evaluate aortic dissection. Often, the extent is sufficiently well presented as to avert the need for catheter angiography before surgery and to plan for aortic stent grafting. Fast helical CT scanners accurately and consistently display the aorta and branch vessels (Fig. 18.1), and advanced multiplanar and three-dimensional reconstructions are important to do this. The CT findings of aortic dissection are listed in Table 18.3. The most characteristic finding is the intimal flap. The typical configuration of the flap is a linear filling defect within the aorta. The intimal flap may also have an atypical configuration: dissection of the entire intima creates a circumferential intimal flap, filiform (extremely narrow) true lumen, a three-channel aorta (Mercedes-Benz sign), and several false channels (Fig. 18.2). The dissection flap is more likely to be curved in an acute dissection and flat in a chronic dissection. CT findings that indicate a ruptured type B dissection include irregularity of the aortic wall, extravasation of vascular contrast material, mediastinal or pericardial hematoma, and hemothorax (1) (Fig. 18.3).
Helical CT with multiplanar reconstructions and magnetic resonance angiography are used for both diagnosis of dissection and surgical or stent-graft planning.
Once the diagnosis of aortic dissection is made, the key information needed by the surgeon is the extent of the dissection and whether branch vessels originate from the true lumen or false lumen. Key features that can be used to distinguish between the true and false lumens are listed in Table 18.4 (Fig. 18.4) (6,7).
The false lumen is often larger than the true lumen, contains cobwebs (strands of media), and is filled with blood that may have slow flow or be thrombosed.
A limitation of CT is the use of intravenous contrast, for which some patients are known to be allergic and which must be avoided in other patients with acute renal failure secondary to dissection or preexisting renal insufficiency. In such settings, MRI is recommended (Table 18.5).
The true lumen gives rise to the coronary arteries and aortic valve and may be surrounded by intimal calcification.
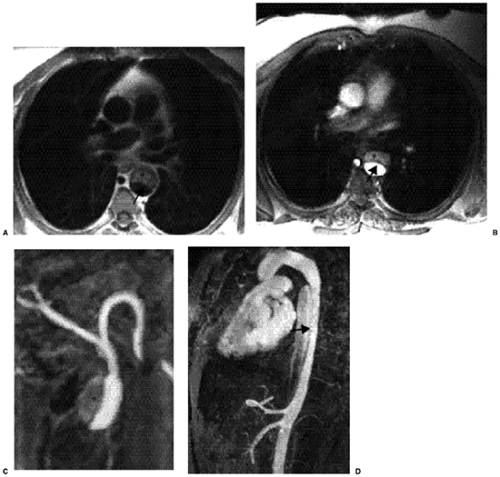
MRI features are similar to those seen on CT. The intensity of signaling of the false lumen is variable and dependent on the blood flow, as well as the age and composition of thrombus. Standard MRI techniques demonstrate pleural and pericardial effusions, mediastinal hemorrhage, and aortic wall thickening (Fig. 18.5).
Table 18.2: Chest Radiograph Findings of Aortic Dissection | |
|---|---|
|
Table 18.3: Computed Tomographic Findings of Aortic Dissection | |
|---|---|
|
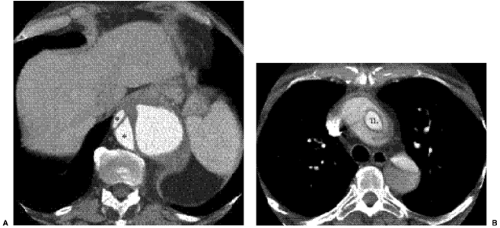 Figure 18.2 Aortic dissection flaps on contrast enhanced computed tomography. A. Multiple false lumens (asterisks). B. Circumferential intimal flap in the ascending thoracic aorta. TL, true lumen. |
Table 18.4: Features to Distinguish True and False Lumens on Computed Tomography | |
|---|---|
|
Table 18.5: MR Imaging Findings of Aortic Dissection | ||
|---|---|---|
|
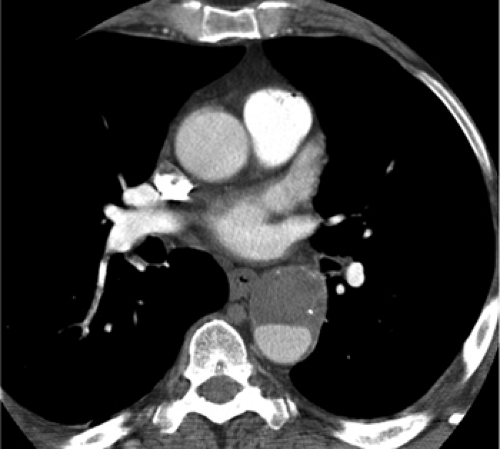 Figure 18.4 Chronic Stanford type B aortic dissection on axial computed tomography with calcification along the peripheral (outer wall) of the thrombosed false lumen. |
Aortography
At aortography dissection appears as an intimal flap or multiple lumens, with opacification of double channels. Linear radial lucency may be seen secondary to the torn or separated intima, with entry and reentry points. Additional findings include a true lumen compressed by the false lumen, a thick aortic wall greater than 5 mm, ulcer-like contrast projections beyond the true lumen, abnormal catheter position away from the lateral aortic border, and arterial branch occlusion.
A thin thrombosed false lumen can result in a normal-appearing aortogram.
Transesophageal Echocardiography
This method can image the entire thoracic aorta, with the exception of the distal transverse arch that is partially obscured by the trachea. Transesophageal echocardiography has the advantage of being performed at the bedside in acutely ill or unstable patients. At transesophageal echocardiography, dissection appears as a mobile linear echo within the aortic lumen. Transesophageal echocardiography also provides information regarding ventricular and aortic valve function, both important in surgical decision making.
Acutely, transesophageal echocardiography can be used for diagnosis at the bedside but is insufficient for surgical planning.
Management
Aortic dissection that involves branch vessels and compromises blood flow is treated based on the mechanism of occlusion. When the dissection flap extends into the lumen of the branch vessel and statically narrows it, treatment is usually angioplasty, with or without the deployment of an intravascular stent. When the dissection flap does not enter the branch vessel but dynamically occludes the vessel by prolapse of the intimal flap into the vessel origin, this obstructs the true lumen above the branch vessel origin and is usually treated with balloon fenestration of the dissection flap, intraaortic endoluminal stent in the true lumen, or both. Additionally, the compressed true lumen may be enlarged, by using an uncovered intraaortic stent (5,8).
Surgical treatment of type A dissection consist of replacing the ascending aorta, aortic root, and aortic valve, reconstructing the aortic root to restore aortic valve competence, and directing blood flow to the true lumen. The coronary arteries are reimplanted into the graft. Mortality rates for surgical treatment range from 10% to 35% (9). Type B dissections are usually treated medically with aggressive antihypertensive control and pain control. For type B dissections treated surgically, a graft is usually placed in the proximal descending thoracic aorta. Early postoperative complications of dissection include myocardial infarction, stroke, respiratory insufficiency, pulmonary embolism, aortic rupture, pseudoaneurysm, and graft infection. Late complications are noted 10 years or more after surgery in 15% to 30% of patients and require reoperation for dilatation of the dissected region to avoid rupture. Reoperation may be required for progressive reduction of myocardial perfusion due to aortic insufficiency (1).
Aortic Dissection Follow-Up
After the diagnosis or repair of an aortic dissection, follow-up imaging is usually performed annually. Complications that may be noted include an aneurysm of the false lumen, with continuous dilatation of the aorta that may result in aortic rupture. An aneurysm of the true lumen may develop, particularly in older hypertensive patients with advanced atherosclerosis. Obstruction or aneurysm of aortic branch vessels may also occur. Postoperatively, pseudoaneurysms may develop at the anastomosis of the graft to the native aorta, further weakening the aorta and usually requiring additional surgery.
Intramural Hematoma
Intramural hematoma was first described by Krutenberg in 1920 as bleeding into the outer layers of the aortic media due to rupture of the vasa vasorum without primary intimal tear, leading to subintimal hemorrhage (10). Intramural hematoma is a variant of aortic dissection, characterized by the absence of both the intimal tear and the direct flow communication between the true and false lumens. It has been postulated that a proximal intramural hematoma may be a precursor or early stage of classic aortic dissection. Acute intramural hematoma has a mortality of 21% (10). Intramural hematoma may progress to a classic aortic dissection, with rupture rates ranging from 32% to 40% for intramural
hematoma confined to the descending aorta and 50% to 100% when it involves the ascending aorta. Hence, emergent surgical repair is usually recommended for intramural hematoma involving the ascending aorta.
hematoma confined to the descending aorta and 50% to 100% when it involves the ascending aorta. Hence, emergent surgical repair is usually recommended for intramural hematoma involving the ascending aorta.
Successful patient outcome with medical therapy has been shown similar to the treatment of type B classic aortic dissection. These patients can be frequently followed with annual noninvasive imaging studies, either CT or MRI. Surgery is performed if a classic aortic dissection or aortic rupture develops (11,12).
Clinical Presentation
Initial presenting symptoms include chest pain (50% to 74%), intrascapular back pain (44% to 84%), and neurologic or vascular complications such as syncope, transient ischemic attack, hoarse voice, paraplegia, mesenteric ischemia, and acute renal failure (13). Patients are usually older than those with classic aortic dissection, with a mean age of 66 years versus 55 years for classic aortic dissection. There is an equal male-to-female ratio for intramural hematoma, compared with the 3:1 ratio for classic aortic dissection. Risk factors for intramural hematoma include hypertension or previous trauma.
Imaging
Intramural hematoma can be diagnosed by CT, MRI, and transesophageal echocardiography. At aortography, it may not be detected due to the inability to opacify a false lumen with contrast in the absence of an entrance tear. This often occurs when the intramural hematoma is thin and does not deform the true lumen.
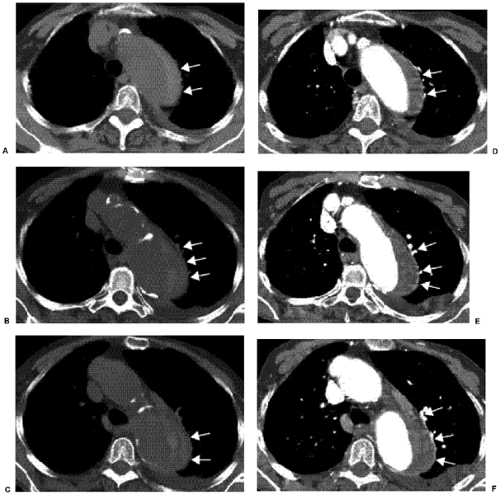
On nonintravenous contrast-enhanced CT, a continuous high attenuation crescent along the aortic wall representing hematoma is usually found without mass effect on the true lumen (Fig. 18.6). As in classic dissection, internally displaced intimal calcification may be seen. At intravenous contrast-enhanced CT, the crescentic area along the aortic wall appears as low attenuation thrombus. There is no intimal flap. The intramural hematoma usually maintains a constant circumferential relationship with the aortic wall. Associated features include pericardial effusion, hemothorax, pleural effusion, and hemomediastinum. Distinguishing an intramural hematoma from classic aortic dissection with a thrombosed false lumen is still problematic.
Acute intramural hematoma is a aortic dissection with no entry tear and appears as a thrombosed false lumen.
At MRI, focal crescentic wall thickening, without mass effect on the aortic lumen and an absence of intimal flap, is seen. In reference to true spin echo (SE) sequences, the signal characteristics of the intramural hematoma depend on the age of the hematoma. If acute, it appears high signal on T2-weighted images and isointense or hypointense to muscle on T1-weighted images due to oxyhemoglobin. When subacute, it is high signal on both T1- and T2-weighted images due to methemoglobin. When chronic, it appears low signal on T1- and T2-weighted images due to organization of the blood (14). At transesophageal echocardiography, intramural hematoma appears as localized circular or crescentic thickening of the aortic wall, greater than or equal to 5 mm, and again, no intimal tear is seen.
Management
Treatment consists of intensive care unit monitoring, aggressive medical treatment with antihypertensive therapy, and frequent serial follow-up noninvasive imaging studies. Surgery is performed in patients with coexistent aneurysmal dilatation or when the intramural hematoma progresses on serial studies. Evaluation of intramural hematoma on serial noninvasive imaging has shown that intramural hematomas may decrease in size and partially or completely resolve, particularly if the aortic diameter is less than 5 cm (12,15). Sometimes ulcer-like projections into the intramural hematoma develop, which can progress to saccular aneurysm. Fusiform aneurysms without ulcer-like projections and even overt aortic dissection can develop (12). Risk factors for overt aortic dissection
include intramural hematoma involving the ascending aorta that had a maximum thickness of 16 mm, compression of the true lumen, and the presence of pericardial or pleural effusion (16).
include intramural hematoma involving the ascending aorta that had a maximum thickness of 16 mm, compression of the true lumen, and the presence of pericardial or pleural effusion (16).
Penetrating Atherosclerotic Ulcer
Penetrating atherosclerotic ulcer is characterized by ulcerating atherosclerotic plaque that penetrates into the internal elastic lamina and media, resulting in hematoma formation within the media of the aortic wall (17,18). Penetrating atherosclerotic ulcer is most often seen in hypertensive elderly patients with a mean age of 70 years. There is a high prevalence of diffuse systemic atherosclerosis and hyperlipidemia. Clinical presentation includes chest or back pain. Less frequently, embolization of atheromatous debris or overlying thrombus results in ischemia and infarction of downstream tissues.
Pathophysiology
Atheromatous ulcers develop in patients with advanced atherosclerosis. At this stage, lesions are usually asymptomatic and confined to the intimal layer. As the lesion progresses and a deep atheromatous ulcer penetrates through the elastic lamina into the media, an intramural hematoma is formed. The hematoma can extend through the adventitia, forming a false aneurysm. Rarely, it may progress to complete transmural aortic rupture (19).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree