THE METABOLIC SYNDROME
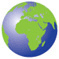
The metabolic syndrome (syndrome X, insulin resistance syndrome) consists of a constellation of metabolic abnormalities that confer increased risk of cardiovascular disease (CVD) and diabetes mellitus (DM). The criteria for the metabolic syndrome have evolved since the original definition by the World Health Organization in 1998, reflecting growing clinical evidence and analysis by a variety of consensus conferences and professional organizations. The major features of the metabolic syndrome include central obesity, hypertriglyceridemia, low high-density lipoprotein (HDL) cholesterol, hyper-glycemia, and hypertension (Table 32-1).
TABLE 32-1
NCEP:ATPIII 2001 AND IDF CRITERIA FOR THE METABOLIC SYNDROME
EPIDEMIOLOGY
 The prevalence of metabolic syndrome varies around the world, in part reflecting the age and ethnicity of the populations studied and the diagnostic criteria applied. In general, the prevalence of metabolic syndrome increases with age. The highest recorded prevalence worldwide is in Native Americans, with nearly 60% of women ages 45–49 and 45% of men ages 45–49 meeting National Cholesterol Education Program and Adult Treatment Panel III (NCEP:ATPIII) criteria. In the United States, metabolic syndrome is less common in African-American men and more common in Mexican-American women. Based on data from the National Health and Nutrition Examination Survey (NHANES) 1999–2000, the age-adjusted prevalence of the metabolic syndrome in U.S. adults who did not have diabetes is 28% for men and 30% for women. In France, a cohort 30 to 60 years old has shown a <10% prevalence for each sex, although 17.5% are affected in the age range 60–64. Greater industrialization worldwide is associated with rising rates of obesity, which is anticipated to increase prevalence of the metabolic syndrome dramatically, especially as the population ages. Moreover, the rising prevalence and severity of obesity in children is initiating features of the metabolic syndrome in a younger population.
The prevalence of metabolic syndrome varies around the world, in part reflecting the age and ethnicity of the populations studied and the diagnostic criteria applied. In general, the prevalence of metabolic syndrome increases with age. The highest recorded prevalence worldwide is in Native Americans, with nearly 60% of women ages 45–49 and 45% of men ages 45–49 meeting National Cholesterol Education Program and Adult Treatment Panel III (NCEP:ATPIII) criteria. In the United States, metabolic syndrome is less common in African-American men and more common in Mexican-American women. Based on data from the National Health and Nutrition Examination Survey (NHANES) 1999–2000, the age-adjusted prevalence of the metabolic syndrome in U.S. adults who did not have diabetes is 28% for men and 30% for women. In France, a cohort 30 to 60 years old has shown a <10% prevalence for each sex, although 17.5% are affected in the age range 60–64. Greater industrialization worldwide is associated with rising rates of obesity, which is anticipated to increase prevalence of the metabolic syndrome dramatically, especially as the population ages. Moreover, the rising prevalence and severity of obesity in children is initiating features of the metabolic syndrome in a younger population.
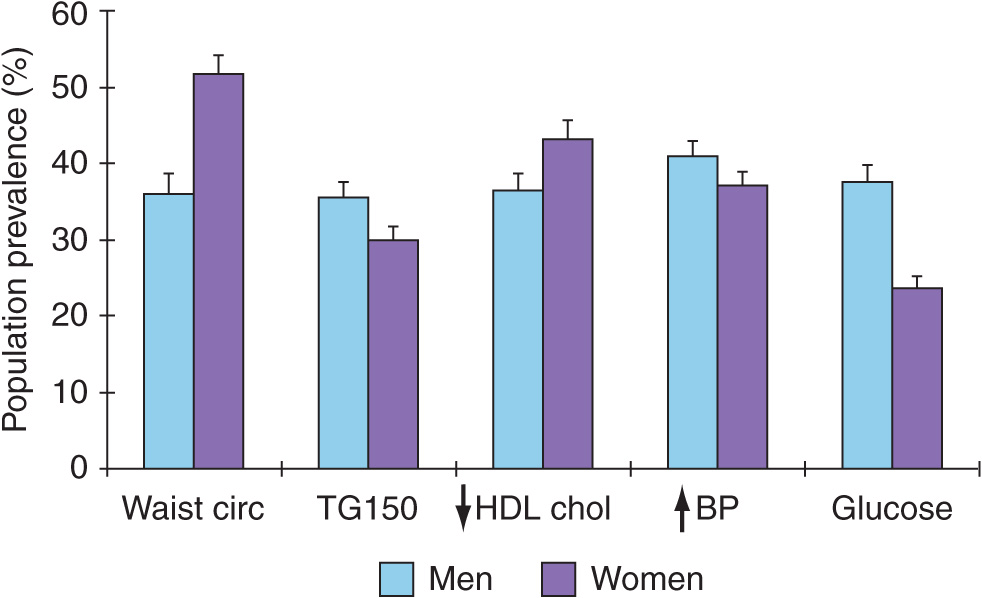
The frequency distribution of the five components of the syndrome for the U.S. population (NHANES III) is summarized in Fig. 32-1. Increases in waist circumference predominate in women, whereas fasting triglycerides >150 mg/dL and hypertension are more likely in men.
FIGURE 32-1
Prevalence of the metabolic syndrome components, from NHANES III. NHANES, National Health and Nutrition Examination Survey; TG, triglyceride; HDL, high-density lipoprotein; BP, blood pressure. The prevalence of elevated glucose includes individuals with known diabetes mellitus. (Created from data in ES Ford et al: Diabetes Care 27:2444, 2004.)
RISK FACTORS
Overweight/obesity
Although the first description of the metabolic syndrome occurred in the early twentieth century, the worldwide overweight/obesity epidemic has been the driving force for more recent recognition of the syndrome. Central adiposity is a key feature of the syndrome, reflecting the fact that the syndrome’s prevalence is driven by the strong relationship between waist circumference and increasing adiposity. However, despite the importance of obesity, patients who are normal weight may also be insulin-resistant and have the syndrome.
Sedentary lifestyle
Physical inactivity is a predictor of CVD events and related mortality rate. Many components of the metabolic syndrome are associated with a sedentary lifestyle, including increased adipose tissue (predominantly central), reduced HDL cholesterol, and a trend toward increased triglycerides, high blood pressure, and increased glucose in the genetically susceptible. Compared with individuals who watched television or videos or used the computer <1 h daily, those who carried out those behaviors for >4 h daily had a twofold increased risk of the metabolic syndrome.
Aging
The metabolic syndrome affects 44% of the U.S. population older than age 50. A greater percentage of women over age 50 have the syndrome than men. The age dependency of the syndrome’s prevalence is seen in most populations around the world.
Diabetes mellitus
DM is included in both the NCEP and International Diabetes Foundation (IDF) definitions of the metabolic syndrome. It is estimated that the great majority (~75%) of patients with type 2 diabetes or impaired glucose tolerance (IGT) have the metabolic syndrome. The presence of the metabolic syndrome in these populations relates to a higher prevalence of CVD compared with patients with type 2 diabetes or IGT without the syndrome.
Coronary heart disease
The approximate prevalence of the metabolic syndrome in patients with coronary heart disease (CHD) is 50%, with a prevalence of 37% in patients with premature coronary artery disease (≤age 45), particularly in women. With appropriate cardiac rehabilitation and changes in lifestyle (e.g., nutrition, physical activity, weight reduction, and, in some cases, pharmacologic agents), the prevalence of the syndrome can be reduced.
Lipodystrophy
Lipodystrophic disorders in general are associated with the metabolic syndrome. Both genetic (e.g., Berardinelli-Seip congenital lipodystrophy, Dunnigan familial partial lipodystrophy) and acquired (e.g., HIV-related lipodys-trophy in patients treated with highly active antiretroviral therapy) forms of lipodystrophy may give rise to severe insulin resistance and many of the components of the metabolic syndrome.
ETIOLOGY
Insulin resistance
The most accepted and unifying hypothesis to describe the pathophysiology of the metabolic syndrome is insulin resistance, which is caused by an incompletely understood defect in insulin action. The onset of insulin resistance is heralded by postprandial hyperinsulinemia, followed by fasting hyperinsulinemia and, ultimately, hyperglycemia.
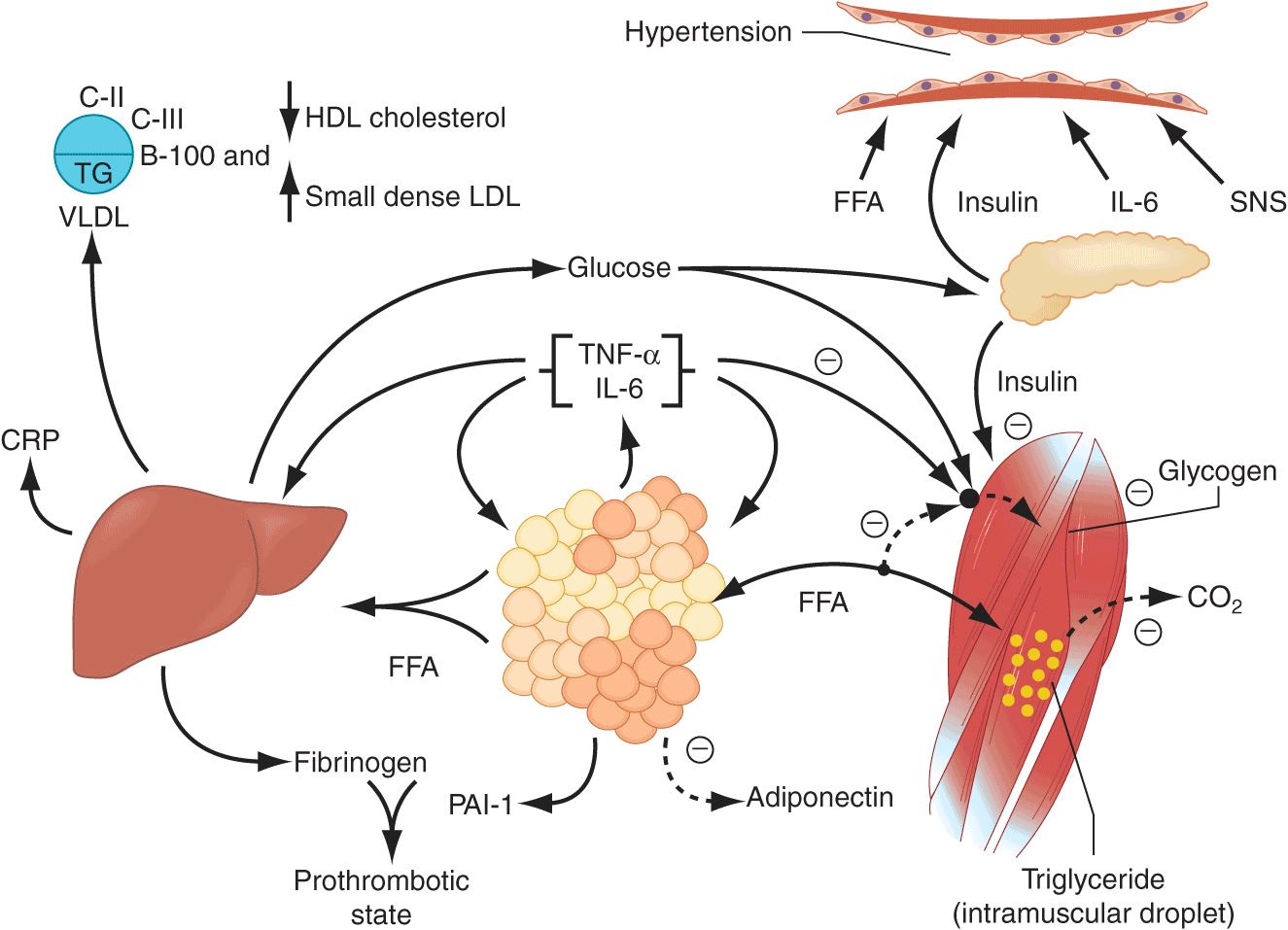
An early major contributor to the development of insulin resistance is an overabundance of circulating fatty acids (Fig. 32-2). Plasma albumin-bound free fatty acids (FFAs) are derived predominantly from adipose tissue triglyceride stores released by lipolytic enzymes lipase. Fatty acids are also derived from the lipolysis of triglyceride-rich lipoproteins in tissues by lipoprotein lipase (LPL). Insulin mediates both antilipolysis and the stimulation of LPL in adipose tissue. Of note, the inhibition of lipolysis in adipose tissue is the most sensitive pathway of insulin action. Thus, when insulin resistance develops, increased lipolysis produces more fatty acids, which further decrease the antilipolytic effect of insulin. Excessive fatty acids enhance substrate availability and create insulin resistance by modifying downstream signaling. Fatty acids impair insulin-mediated glucose uptake and accumulate as triglycerides in both skeletal and cardiac muscle, whereas increased glucose production and triglyceride accumulation are seen in the liver.
FIGURE 32-2
Pathophysiology of the metabolic syndrome. Free fatty acids (FFAs) are released in abundance from an expanded adipose tissue mass. In the liver, FFAs result in an increased production of glucose and triglycerides and secretion of very low-density lipoproteins (VLDLs). Associated lipid/lipoprotein abnormalities include reductions in high-density lipoprotein (HDL) cholesterol and an increased density of low-density lipoproteins (LDLs). FFAs also reduce insulin sensitivity in muscle by inhibiting insulin-mediated glucose uptake. Associated defects include a reduction in glucose partitioning to glycogen and increased lipid accumulation in triglyceride (TG). Increases in circulating glucose, and to some extent FFA, increase pancreatic insulin secretion, resulting in hyper-insulinemia. Hyperinsulinemia may result in enhanced sodium reabsorption and increased sympathetic nervous system (SNS) activity and contribute to the hypertension, as might increased levels of circulating FFAs. The proinflammatory state is superimposed and contributory to the insulin resistance produced by excessive FFAs. The enhanced secretion of interleukin 6 (IL-6) and tumor necrosis factor α(TNF-α) produced by adipocytes and monocyte-derived macrophages results in more insulin resistance and lipolysis of adipose tissue triglyceride stores to circulating FFAs. IL-6 and other cytokines also enhance hepatic glucose production, VLDL production by the liver, and insulin resistance in muscle. Cytokines and FFAs also increase the hepatic production of fibrinogen and adipocyte production of plasminogen activator inhibitor 1 (PAI-1), resulting in a prothrombotic state. Higher levels of circulating cytokines also stimulate the hepatic production of C-reactive protein (CRP). Reduced production of the anti-inflammatory and insulin-sensitizing cytokine adiponectin is also associated with the metabolic syndrome. (Reprinted from RH Eckel et al: Lancet 365:1415, 2005, with permission from Elsevier.)
The oxidative stress hypothesis provides a unifying theory for aging and the predisposition to the metabolic syndrome. In studies carried out in insulin-resistant subjects with obesity or type 2 diabetes, the offspring of patients with type 2 diabetes, and the elderly, a defect has been identified in mitochondrial oxidative phosphorylation, leading to the accumulation of triglycerides and related lipid molecules in muscle. The accumulation of lipids in muscle is associated with insulin resistance.
Increased waist circumference
Waist circumference is an important component of the most recent and frequently applied diagnostic criteria for the metabolic syndrome. However, measuring waist circumference does not reliably distinguish increases in subcutaneous adipose tissue vs. visceral fat; this distinction requires CT or MRI. With increases in visceral adipose tissue, adipose tissue-derived FFAs are directed to the liver. In contrast, increases in abdominal subcutaneous fat release lipolysis products into the systemic circulation and avoid more direct effects on hepatic metabolism. Relative increases in visceral versus subcutaneous adipose tissue with increasing waist circumference in Asians and Asian Indians may explain the greater prevalence of the syndrome in those populations compared with African-American men in whom subcutaneous fat predominates. It is also possible that visceral fat is a marker for, but not the source of, excess postprandial FFAs in obesity.
Dyslipidemia
(See also Chap. 31) In general, FFA flux to the liver is associated with increased production of apoB-containing, triglyceride-rich very low-density lipoproteins (VLDLs). The effect of insulin on this process is complex, but hypertriglyceridemia is an excellent marker of the insulin-resistant condition.
The other major lipoprotein disturbance in the metabolic syndrome is a reduction in HDL cholesterol. This reduction is a consequence of changes in HDL composition and metabolism. In the presence of hypertriglyceridemia, a decrease in the cholesterol content of HDL is a consequence of reduced cholesteryl ester content of the lipoprotein core in combination with cholesteryl ester transfer protein–mediated alterations in triglyceride, making the particle small and dense. This change in lipoprotein composition also results in increased clearance of HDL from the circulation. The relationships of these changes in HDL to insulin resistance are probably indirect, occurring in concert with the changes in triglyceride-rich lipoprotein metabolism.
In addition to HDL, low-density lipoproteins (LDLs) are modified in composition. With fasting serum triglycerides >2.0 mM (~180 mg/dL), there is almost always a predominance of small dense LDLs. Small dense LDLs are thought to be more atherogenic. They may be toxic to the endothelium, and they are able to transit through the endothelial basement membrane and adhere to glycosaminoglycans. They also have increased susceptibility to oxidation and are selectively bound to scavenger receptors on monocyte-derived macrophages. Subjects with increased small dense LDL particles and hypertriglyceridemia also have increased cholesterol content of both VLDL1 and VLDL2 subfractions. This relatively cholesterol-rich VLDL particle may contribute to the atherogenic risk in patients with metabolic syndrome.
Glucose intolerance
The defects in insulin action lead to impaired suppression of glucose production by the liver and kidney and reduced glucose uptake and metabolism in insulin-sensitive tissues, i.e., muscle and adipose tissue. The relationship between impaired fasting glucose (IFG) or impaired glucose tolerance (IGT) and insulin resistance is well supported by human, nonhuman primate, and rodent studies. To compensate for defects in insulin action, insulin secretion and/or clearance must be modified to sustain euglycemia. Ultimately, this compensatory mechanism fails, usually because of defects in insulin secretion, resulting in progress from IFG and/or IGT to DM.
Hypertension
The relationship between insulin resistance and hypertension is well established. Paradoxically, under normal physiologic conditions, insulin is a vasodilator with secondary effects on sodium reabsorption in the kidney. However, in the setting of insulin resistance, the vasodilatory effect of insulin is lost but the renal effect on sodium reabsorption is preserved. Sodium reabsorption is increased in whites with the metabolic syndrome but not in Africans or Asians. Insulin also increases the activity of the sympathetic nervous system, an effect that also may be preserved in the setting of the insulin resistance. Finally, insulin resistance is characterized by pathway-specific impairment in phosphatidylinositol-3-kinase signaling. In the endothelium, this may cause an imbalance between the production of nitric oxide and the secretion of endothelin 1, leading to decreased blood flow. Although these mechanisms are provocative, when insulin action is assessed by levels of fasting insulin or by the Homeostasis Model Assessment (HOMA), insulin resistance contributes only modestly to the increased prevalence of hypertension in the metabolic syndrome.
Proinflammatory cytokines
The increases in proinflammatory cytokines, including interleukin (IL)-1, IL-6, IL-18, resistin, tumor necrosis factor (TNF) α, and C-reactive protein (CRP), reflect overproduction by the expanded adipose tissue mass (Fig. 32-2). Adipose tissue-derived macrophages may be the primary source of proinflammatory cytokines locally and in the systemic circulation. It remains unclear, however, how much of the insulin resistance is caused by the paracrine vs. endocrine effects of these cytokines.
Adiponectin
Adiponectin is an anti-inflammatory cytokine produced exclusively by adipocytes. Adiponectin enhances insulin sensitivity and inhibits many steps in the inflammatory process. In the liver, adiponectin inhibits the expression of gluconeogenic enzymes and the rate of glucose production. In muscle, adiponectin increases glucose transport and enhances fatty acid oxidation, partially due to activation of adenosine monophosphate (AMP) kinase. Adiponectin is reduced in the metabolic syndrome. The relative contribution of adiponectin deficiency versus overabundance of the proinflammatory cytokines is unclear.
CLINICAL FEATURES
Symptoms and signs
The metabolic syndrome is typically not associated with symptoms. On physical examination, waist circumference may be expanded and blood pressure elevated. The presence of one or either of these signs should alert the clinician to search for other biochemical abnormalities that may be associated with the metabolic syndrome. Less frequently, lipoatrophy or acanthosis nigricans is found on examination. Because these physical findings typically are associated with severe insulin resistance, other components of the metabolic syndrome should be expected.
Associated diseases
 Cardiovascular disease
Cardiovascular disease
The relative risk for new-onset CVD in patients with the metabolic syndrome, in the absence of diabetes, averages between 1.5-fold and threefold. However, in an 8-year follow-up of middle-aged men and women in the Framingham Offspring Study (FOS), the population-attributable risk for patients with the metabolic syndrome to develop CVD was 34% in men and only 16% in women. In the same study, both the metabolic syndrome and diabetes predicted ischemic stroke, with greater risk for patients with the metabolic syndrome than for those with diabetes alone (19% vs 7%), particularly in women (27% vs 5%). Patients with metabolic syndrome are also at increased risk for peripheral vascular disease.
 Type 2 diabetes
Type 2 diabetes
Overall, the risk for type 2 diabetes in patients with the metabolic syndrome is increased three- to fivefold. In the FOS’s 8-year follow-up of middle-aged men and women, the population-attributable risk for developing type 2 diabetes was 62% in men and 47% in women.
Other associated conditions
In addition to the features specifically associated with metabolic syndrome, insulin resistance is accompanied by other metabolic alterations. Those alterations include increases in apoB and apoC-III, uric acid, prothrombotic factors (fibrinogen, plasminogen activator inhibitor 1), serum viscosity, asymmetric dimethylarginine, homocysteine, white blood cell count, proinflammatory cytokines, CRP, microalbuminuria, nonalcoholic fatty liver disease (NAFLD) and/or nonalcoholic steatohepatitis (NASH), polycystic ovarian disease (PCOS), and obstructive sleep apnea (OSA).
 Nonalcoholic fatty liver disease
Nonalcoholic fatty liver disease
Fatty liver is relatively common. However, in NASH, both triglyceride accumulation and inflammation coexist. NASH is now present in 2–3% of the population in the United States and other Western countries. As the prevalence of overweight/obesity and the metabolic syndrome increases, NASH may become one of the more common causes of end-stage liver disease and hepatocellular carcinoma.
 Hyperuricemia
Hyperuricemia
Hyperuricemia reflects defects in insulin action on the renal tubular reabsorption of uric acid, whereas the increase in asymmetric dimethylarginine, an endogenous inhibitor of nitric oxide synthase, relates to endothelial dysfunction. Microalbuminuria also may be caused by altered endothelial pathophysiology in the insulin-resistant state.
 Polycystic ovary syndrome
Polycystic ovary syndrome
PCOS is highly associated with the metabolic syndrome, with a prevalence between 40% and 50%. Women with PCOS are two to four times more likely to have the metabolic syndrome than are women without PCOS.
 Obstructive sleep apnea
Obstructive sleep apnea
OSA is commonly associated with obesity, hypertension, increased circulating cytokines, IGT, and insulin resistance. With these associations, it is not surprising that the metabolic syndrome is frequently present. Moreover, when biomarkers of insulin resistance are compared between patients with OSA and weight-matched controls, insulin resistance is more severe in patients with OSA. Continuous positive airway pressure (CPAP) treatment in OSA patients improves insulin sensitivity.
DIAGNOSIS
The diagnosis of the metabolic syndrome relies on satisfying the criteria listed in Table 32-1 by using tools at the bedside and in the laboratory. The medical history should include evaluation of symptoms for OSA in all patients and PCOS in premenopausal women. Family history will help determine risk for CVD and DM. Blood pressure and waist circumference measurements provide information necessary for the diagnosis.
Laboratory tests
Fasting lipids and glucose are needed to determine if the metabolic syndrome is present. The measurement of additional biomarkers associated with insulin resistance can be individualized. Such tests might include apoB, high-sensitivity CRP, fibrinogen, uric acid, urinary microalbumin, and liver function tests. A sleep study should be performed if symptoms of OSA are present. If PCOS is suspected on the basis of clinical features and anovulation, testosterone, luteinizing hormone, and follicle-stimulating hormone should be measured.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree