The Evaluation and Management of Chronic Ischemic Heart Disease: Introduction
Chronic ischemic heart disease from coronary artery atherosclerosis remains a chief public health concern in most industrialized nations and has become a leading cause of death and disability in developing countries.1 Thrombosis complicating an atherosclerotic plaque is the proximate cause of acute myocardial infarction (MI) in patients with coronary artery disease (CAD) and represents the leading cause of death for men and women worldwide.2 As a result of improved survival rates following MI, the size of the patient population living with chronic CAD is increasing. For example, in the United States, it is estimated that 470,000 individuals suffer a recurrent MI yearly.3 In these patients, the transition from a clinically stable coronary syndrome to an acute life-threatening event remains largely unpredictable despite substantial gains in the understanding of the pathobiology of atherosclerosis.4 Traditional (eg, history) and contemporary (eg, advanced imaging) methods of diagnosis and risk stratification are used to initiate treatment strategies. This chapter provides a framework for the evaluation and management of patients with chronic CAD. Conclusions from landmark clinical trials that have influenced the application of medical, percutaneous, and surgical treatments will be discussed.
Overview of Chronic Ischemic Heart Disease
Myocardial ischemia is mediated by an imbalance between oxygen supply and demand at the cellular (myocyte) level (see Chap. 54). Coronary atherosclerosis impairs coronary blood flow (CBF) via a variety of mechanisms and is the dominant cause of angina under conditions of elevated myocardial oxygen demand, such as exercise or emotional stress. CBF is impaired in several other disease states, including severe aortic valve disease with left ventricular hypertrophy (LVH), hypertension, idiopathic dilated cardiomyopathy, and hypertrophic cardiomyopathy, even in the absence of epicardial CAD. In patients with LVH, ischemia may result from a combination of inadequate capillary density, pathologic changes within small intramyocardial arteries and arterioles, reduced CBF reserve, systolic compressive forces, and markedly elevated diastolic pressures within the vulnerable subendocardium. A primary reduction in myocardial oxygen supply following intraluminal thrombus formation and/or epicardial constriction underlies the development of acute coronary syndromes. Reduced oxygen supply in the chronic setting may derive from severe anemia or hemoglobin disorders. The major determinants of myocardial oxygen demand, namely heart rate, wall stress, and contractility, can singularly or in combination trigger an ischemic cascade in a vulnerable patient.
For over two centuries, it has been recognized that cardiac angina can be effectively diagnosed by a careful patient interview. William Herberden is credited with the initial description of angina in 1772 in his chapter entitled, “Pectoris Dolor,” from his Commentaries on the History and Cure of Diseases.5 Remarkably, several elements from this original characterization remain pertinent to the management of CAD today. Herberden correctly identified that certain descriptive features of angina, such as the occurrence of chest pain at rest, portend a particularly grave prognosis.
The Canadian Cardiovascular Society (CCS) classification, now more than 3 decades old, is the most commonly used system for grading angina severity in use today (Table 64–1).6 Increasing CCS class positively correlates with the number of diseased epicardial coronary arteries discovered at angiography, the presence of impaired left ventricular (LV) function, and subsequent rates of percutaneous coronary intervention (PCI) or bypass surgery.7 The CCS classification system can also be used to predict outcome in patients with chronic CAD. Compared with CCS class I status, CCS class IV status is associated with significantly elevated rates of all-cause mortality and nonfatal MI 30 months following revascularization.7 In this and other published analyses, the prognostic strength of CCS class is maintained even after statistical adjustment for conventional CAD-associated risk factors, including smoking status and diabetes. Shortcomings associated with the CCS scale include only a modest correlation with exercise stress test results and mixed reports regarding inter-rater reliability.6,8
| I | II | III | IV |
|---|---|---|---|
Ordinary physical activity does not cause angina including: Walking and climbing stairs Angina occurs: Only with strenuous, rapid, or prolonged exertion at work or recreation | Slight limitation of ordinary activity including: Walking stairs rapidly Walking uphill Stair climbing after meals Angina occurs: A few hours after awakening Walking > 2 city blocks (level ground) Walking 1 flight of ordinary stairs at a normal pace | Marked limitation of ordinary physical activity. Angina occurs: Walking ≤ 1 city block (level ground) Climbing one flight of stairs under normal conditions and at a normal pace | Inability to perform any physical activity without discomfort. Angina occurs: With minimal activity May be present at rest |
Myocardial ischemia is characterized as asymptomatic, or silent, when it occurs in the absence of angina or an anginal equivalent.9 When present, asymptomatic ischemia is positively correlated with incident ischemic heart disease and confers an unfavorable prognosis.10 The pathophysiology of asymptomatic ischemia remains controversial. Leading contemporary theories emphasize the presence of defective sensory afferent nerve function, which impairs normal sensory conduction from the atria and ventricles to the thoracic sympathetic ganglia and dorsal roots of the spinal cord. Asymptomatic ischemia is frequently observed among patients with diabetes, presumably because of the associated neuropathy. Other patient populations at risk for asymptomatic ischemia include those with a previous MI. Cohn and colleagues9 estimate that 50,000 patients per year experience an asymptomatic MI within 30 days of an initial event. Asymptomatic ischemia occurring during the hospital phase of MI is predictive of future cardiovascular events over the next 5 years.9 In selected patients with chronic CAD, the prognostic value of asymptomatic ischemia on treadmill exercise stress testing is similar to that associated with symptomatic ischemia, particularly when present at low workloads.11,12 The influence of asymptomatic ischemia on clinical management strategies beyond conventional pharmacotherapy is unresolved because there are currently insufficient data to support coronary revascularization based on the presence of asymptomatic ischemia alone, although the magnitude and extent of asymptomatic ischemia may appropriately influence invasive treatment decisions in individual patients.
Impaired LV systolic function is the primary cardiovascular form of end-organ damage in patients with chronic CAD. Systolic heart failure (HF) is most likely to occur under conditions in which ≥20% of myocardium is injured from chronic ischemia or prior MI(s). When present, replacement fibrosis following myocyte injury and LV remodeling lead to unfavorable changes in contractile performance, stroke volume, and cardiac output. Mitral regurgitation may emerge as a consequence of remodeling. Collectively, these changes result in maladaptive neurohormonal signaling, which is implicated in the perpetuation of further cardiovascular dysfunction. Ischemic HF may occur alone or in combination with other mechanisms. Mitral regurgitation from ischemia-mediated LV remodeling occurs as a consequence of papillary muscle displacement, leaflet tethering, and/or annular dilatation and is strongly associated with HF and poor long-term outcomes in a graded fashion. Controversy exists regarding the optimal management of patients with ischemic mitral regurgitation who are referred for coronary artery bypass graft surgery (CABG). Randomized trials are underway to investigate this issue. Atrial fibrillation is the most common arrhythmia among patients with ischemic HF; ventricular tachycardia is of greatest clinical concern. Sustained monomorphic ventricular tachycardia may arise in or near an area of myocardial scar formation, whereas polymorphic ventricular tachycardia may occur as a consequence of acute ischemia, electrolyte imbalance, or drug effect.
Patients with atrial fibrillation (see Chap. 40) or those with specific acquired forms of structural heart disease (eg, LV aneurysm or LV apical akinesis) are at increased risk for systemic thromboembolism. Anticoagulant therapy is indicated for stroke prevention according to the patient’s individualized risk profile and in alignment with published clinical management guidelines.
Factors that Promote the Progression of Chronic CAD
Once believed primarily to reflect an abnormality in cholesterol storage, it is now recognized that coronary atherosclerotic plaque formation occurs as a consequence of aberrant molecular signaling pathways that increase vascular inflammation, induce injury to blood vessel wall cellular components, and promote negative vascular remodeling.13 An understanding of the cellular mechanisms that contribute to atherogenesis has helped focus efforts on imaging modalities for detection and characterization of CAD. Electron-beam computed tomography (EBCT) is very sensitive for the detection of coronary calcification, the imaging signature of chronic atherosclerosis.14 Molecular imaging techniques for quantitative evaluation of plaque inflammation may help identify at-risk lesions in vulnerable patients. Catheter-based infrared fluorescence, plaque thermography, contrast-enhanced magnetic resonance imaging (MRI), and fluorodeoxyglucose positron emission tomography (PET) hold promise for real-time diagnosis and enhanced characterization of CAD.15,16
Predicting future cardiovascular events in patients with CAD remains a difficult task. The identification of specific patients who are at elevated risk for acute MI due to the presence of certain atherosclerotic lesion characteristics and/or clinical factors is a central component of contemporary evaluation and management strategies. Ongoing research is focused on discriminating at-risk atherosclerotic lesions based on propensity for rupture, rapid progression in size, or the expression of other features that suggest a high likelihood for future thrombotic complications.15 Collectively, these characteristics describe a vulnerable plaque, a lesion the attributes of which predict a higher likelihood of thrombosis, MI, and/or ventricular arrhythmias. A uniformly accepted definition of the vulnerable plaque is unresolved, but early recommendations have provided major and minor criteria for categorization of lesions at elevated risk (Table 64–2). These criteria reflect anatomic, morphologic, molecular, and physiologic features and may ultimately assist clinicians in treatment decisions.
| Major criteria |
| Active inflammation (eg, monocyte, macrophage, ± T-cell infiltration) |
| Thin cap with large lipid core |
| Endothelial denudation with superficial platelet aggregation |
| Fissured plaque |
| Luminal stenosis >90% |
| Minor criteria |
| Superficial calcified nodule |
| Glistening yellow appearance (pathologic diagnosis) |
| Intraplaque hemorrhage |
| Outward remodeling |
| Endothelial dysfunction |
The vulnerable plaque concept has been expanded in recent years to include vulnerable patients at risk for an acute coronary syndrome (ACS) or sudden cardiac death (SCD) based on their plaque, hematologic, and/or myocardial substrate. Examples include patients with CAD and hypercoagulable disorders and patients with certain forms of cardiomyopathy that predispose to electrical-mechanical instability and unstable arrhythmias.13,15
There is a great deal of overlap between conventional risk factors associated with the de novo development of atherosclerosis and those that promote progression of existing disease. Identifying treatable risk factors in patients with chronic CAD should be accomplished as recommended by the American College of Cardiology (ACC)/American Heart Association (AHA) guidelines for secondary prevention and cardiac rehabilitation (see Chap. 51).17 These guidelines call for a nine-point strategy focused on specific biochemical, psychosocial, and exercise training–associated goals to decrease the progression of chronic CAD and its manifestations (Table 64–3). Interventions that decrease the incidence of ischemic events include those that lead to reductions in cigarette smoking, low-density lipoprotein (LDL) cholesterol, systemic hypertension, LVH, and factors that promote thrombosis.
| Core Component | Objective |
|---|---|
| Patient assessment | Medical history: Review cardiovascular, medical, and surgical diagnoses, comorbidities, symptoms of cardiovascular disease, and medications. Physical examination: Assess cardiopulmonary systems, postcardiovascular procedure wound sites, orthopedic and neuromuscular status, and cognitive function. Testing: ECG testing should occur when appropriate. |
| Nutritional counseling | Evaluation: Assess daily caloric intake, dietary content of saturated fat, trans fat, cholesterol, sodium, and nutrients. Evaluate dining habits. Weight management: For patients with body mass index >25 kg/m2 and/or waist >102 cm in men and 88 cm in women, establish short- and long-term weight reduction goals (eg, 1-2 lb/wk over 6 mo to achieve ≥5% reduction in body weight). |
| Blood pressure management | Evaluation: Measure seated resting blood pressure at ≥2 visits and in both arms. Intervention: For blood pressure 120-139/80-89 mm Hg, recommend key lifestyle modifications (eg, weight loss, alcohol consumption reduction). For blood pressure ≥130/≥80 mm Hg, pharmacotherapy may be appropriate in addition to lifestyle modification in the setting of comorbid chronic kidney disease, heart failure, or diabetes. For blood pressure ≥140 mm Hg systolic or ≥90 mm Hg diastolic, recommend lifestyle modification and pharmacotherapy. |
| Lipid management | Evaluation: Obtain fasting measures of total cholesterol, high-density lipoprotein, low-density lipoprotein, and triglycerides. Evaluate lipid-lowering medication compliance. Intervention: Initiate or intensify lipid-lowering therapy in patients with a measured low-density lipoprotein level >100 mg/dL, or >70 mg/dL if enhanced therapy can be safely provided. Initiate interventions directed toward management of triglycerides to attain non–high-density lipoprotein cholesterol <130 mg/dL. |
| Diabetes management | Evaluation: Confirm presence or absence of diabetes. For patients with diabetes, educate patients regarding signs and symptoms suggestive of hyperglycemia or hypoglycemia. Intervention: Lifestyle modification and diabetes treatment should occur in accordance with recommendations outlined by the American Diabetes Association.a |
| Tobacco cessation | Evaluation: Assess smoking status and use of other tobacco products. Determine readiness to change tobacco use habits. Intervention: If readiness to change tobacco habits is not expressed, provide motivational message. If readiness to change is expressed, individual or group counseling, pharmacologic, or supplemental strategies (eg, hypnosis) may be appropriate. |
| Psychosocial management | Evaluation: Identify the presence of psychological distress (eg, depression) or the use of psychotropic medications. Intervention: Offer individual or group education and counseling on the adjustment to heart disease. Refer patients with clinically significant psychosocial distress to appropriate mental health specialists for further evaluation. |
| Physical activity | Evaluation: Assess current physical activity level and readiness to change physical activity behaviors. Intervention: Encourage patients to perform 30-60 min/d of moderate-intensity physical activity on ≥5 d/wk. Caution patients to avoid performing unaccustomed vigorous physical activity. |
| Exercise training | Evaluation: A symptom-limited exercise test should be performed prior to participation in an exercise-based cardiac rehabilitation program. Intervention: Develop an individualized exercise prescription for aerobic and resistance training that is based on evaluation findings, risk stratification, and comorbidities. |
Numerous observational studies have demonstrated a positive, continuous, and graded relationship between systemic blood pressure and cardiovascular disease risk.18,19 In chronic ischemic heart disease patients, hypertension is a risk factor for recurrent MI, an observation that is likely a consequence of the associated endothelial dysfunction and the adverse effects of persistently elevated afterload on myocardial function and oxygen demand. In patients with diabetes, uncontrolled hypertension is a strong predictor of premature death, cardiovascular morbidity, and progressive nephropathy. The Joint National Committee on Prevention (JNC VII) guidelines recommend a systemic blood pressure of ≤130/85 mm Hg in patients with known CAD and other cardiovascular risk factors such as diabetes or chronic kidney disease (see Chap. 70).20
The presence of LVH confers an increased risk for incident MI, HF, and SCD in patients with ischemic heart disease. It has been proposed that the risk imposed by LVH may be reversible. For example, electrocardiographic (ECG) evidence of LVH regression in treated hypertensive patients is associated with a significant reduction in cardiovascular disease event rates.21
In addition to the conventional risk factors associated with coronary heart disease events discussed earlier, novel associations between environmental risk factors and the progression of ischemic heart disease have been identified. Gerber and colleagues22 demonstrated that low socioeconomic status and limited access to health care confer a significantly elevated risk for adverse long-term (~10 years) outcome in post-MI patients.22 Chronic exposure to air pollution (ie, fine particulate pollution) is also associated with an increased risk of coronary heart disease mortality that is believed to occur as a consequence of increased vascular inflammation or dysregulation of cardiovascular autonomic function.23
The role of substance abuse in the development and progression of CAD is increasingly recognized. The adverse effects of tobacco use on the cardiovascular system are well established. It is estimated that tobacco use alone is responsible approximately 435,000, or 18%, of all deaths in the United States annually.24 Perhaps less well recognized is the prevalence of recreational cocaine use and its contribution to coronary heart disease. Nearly 34 million people in the United States have reported a history of cocaine use, of whom approximately 1% report use within the last month.25 Cocaine can accelerate coronary atherosclerosis, enhance platelet aggregation, induce coronary vasospasm, and increase myocardial oxygen demand via its effects on heart rate and blood pressure.26
Treatment of several noncardiac medical conditions, such as various malignancies, may also predispose to coronary heart disease events in vulnerable populations. Mantle radiation for the treatment of Hodgkin lymphoma is associated with a significantly increased incidence of proximal CAD, higher coronary artery calcification scores (see Coronary Artery Calcification Score in Ischemic Heart Disease section), and an elevated likelihood of the need for revascularization therapy.27 Several chemotherapeutic agents, particularly fluorouracil and capecitabine, may precipitate angina at the microvascular level. Although patients with orthotropic heart transplantations may develop epicardial CAD, graft failure long term is most commonly due to a diffuse, small-vessel arteriopathy that is immune mediated.28 Patients infected with the human immunodeficiency virus (HIV) appear to be at increased risk for a wide range of inflammatory vascular diseases, including CAD. This may occur as a manifestation of HIV itself or as a consequence of highly active antiretroviral therapy (HAART).29 Although the extent to which HAART therapy promotes coronary atherosclerosis is unresolved, some have speculated that HAART may increase the risk of incident MI by four-fold among HIV patients.30 The mechanistic link between HAART and CAD remains speculative but may involve cross-reactivity between protease inhibitors and lipid metabolism-regulating proteins that results in dyslipidemia, insulin resistance, and lipodystrophy.29,31 In selected HIV patients at elevated risk for cardiovascular disease, protease inhibitors may be inappropriate; under these circumstances, consultation with an infectious disease specialist to determine optimal HIV therapy is always advised.
Diagnostic Testing in Chronic CAD
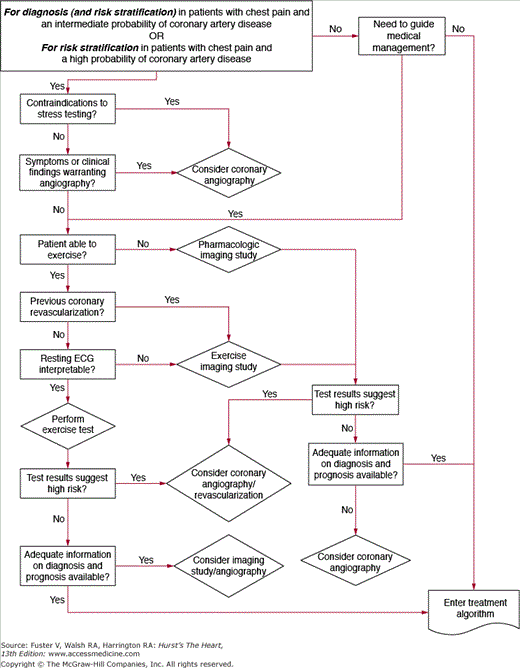
Technologic advances in the past 2 decades have expanded the noninvasive options available for diagnosis and risk stratification of patients with chronic CAD. Although guidelines and appropriate use criteria have been published, practice patterns vary widely in the application of testing to the individual patient. Noninvasive testing, with or without imaging as dictated by specific patient attributes, is appropriate for the vast majority of patients. There are high-risk clinical features, however, that would substantiate the use of invasive coronary angiography as the first step (Fig. 64–1).
Figure 64–1.
Flow chart demonstrating the recommendations for invasive angiography for diagnosis or risk stratification of coronary artery disease. ECG, electrocardiogram. Reproduced with permission from Gibbons RJ, Chatterjee K, Daley J, et al. ACC/AHA/ACP-ASIM guidelines for the management of patients with chronic stable angina: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee on Management of Patients with Chronic Stable Angina). J Am Coll Cardiol. 1999;33:2092-2197.
Numerous studies involving thousands of patients have validated the prognostic utility of ECG treadmill testing. Data from patients with medically managed chronic CAD enrolled in the Coronary Artery Surgery Study (CASS) demonstrated that failure to complete stage 1 of a standard Bruce protocol was associated with a markedly elevated mortality risk 4 years after testing.11 Others have shown that the presence of exercise-induced ST-segment depression, an abnormal blood pressure response to exercise, and poor functional capacity on stress testing is associated with early mortality from cardiovascular disease.32
The Duke Treadmill Score (DTS) is commonly used in clinical practice for predicting the probability of a future MI or cardiovascular death in patients with CAD. In this model, a numeric score is generated that reflects patient exercise time, extent of ST-segment depression, and presence of anginal symptoms. The DTS correlates with future cardiovascular mortality; is valid in the era of diuretic, renin-angiotensin-aldosterone axis inhibitor, and β-adrenergic receptor antagonist therapies; and is predictive in both men and women.33 The DTS and other similar models that use functional capacity and ECG findings as the chief performance measures may be confounded by baseline ECG abnormalities and limitations to exercise from noncardiovascular causes. These scoring systems do not include information regarding the anatomic distribution, extent, and severity of ischemia, nor do they involve an assessment of LV function during exercise stress. Access to these data often enhances the prognostic valve of stress testing and provides the clinician with a more informed understanding of ischemic burden from which to guide treatment decisions in specific patients.34
Resting transthoracic echocardiography is useful to characterize LV systolic and diastolic function in patients with chronic CAD, especially in those with prior MI and HF. Reduced LV ejection fraction (EF) is strongly associated with an increased risk for incident ventricular tachycardia and SCD. In select patients following MI, low EF alone (≤ 0.30) is sufficient to warrant certain forms of device therapy such as an implantable cardioverter-defibrillator. Stress echocardiography is more sensitive and specific than ECG stress for the detection of CAD. In patients with chronic CAD, the presence of LVH, diminution in systolic wall thickening in one or more segments during stress, or compensatory hyperkinesis in nonischemic segments portends increased risk for future cardiovascular events.35,36
Myocardial perfusion imaging (MPI) typically involves single-photon emission computed tomography (SPECT) with visual and quantitative analysis. Attenuation correction algorithms improve test performance. ECG gating is used for analysis of global and regional LV performance. Table 64–4 summarizes the comparative advantages of stress MPI and stress echocardiography for CAD diagnosis. A normal stress MPI study in a patient with an ischemic ECG response to exercise defines a favorable prognosis for which an invasive treatment strategy is usually not justified.37
| Advantages of stress echocardiography |
| 1. Higher specificity |
| 2. Versatility; more extensive evaluation of cardiac anatomy and function |
| 3. Greater convenience/efficacy/availability |
| 4. Lower cost |
| Advantages of stress perfusion imaging |
| 1. Higher technical success rate |
| 2. Higher sensitivity, especially for single-vessel coronary disease involving the left circumflex artery |
| 3. Better accuracy for evaluating possible ischemia when multiple resting left ventricular wall motion abnormalities are present |
| 4. More extensive published database, especially in evaluation of prognosis |
Abnormal stress MPI in patients with established CAD is predictive of cardiac death and nonfatal MI. Transient, stress-induced LV dilation or a decrease in EF of >5% are independent predictors of cardiac death and are useful in risk stratification of patients with chronic CAD.38
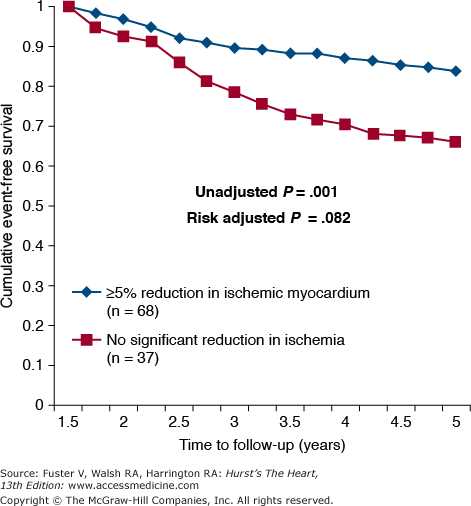
MPI data are useful for identifying high-risk chronic stable CAD patients for whom revascularization therapy may be appropriate.39 Table 64–5 summarizes the MPI and echocardiographic stress test indicators of high, intermediate, and low risk. Referral for angiography to search for CAD extensive enough to warrant surgical revascularization is reasonable for patients with high-risk features. A subset analysis of 314 patients from the Clinical Outcomes Utilizing Revascularization and Aggressive Drug Evaluation (COURAGE) trial suggested that adding PCI to optimal medical therapy (OMT) may be more efficacious than OMT alone for the treatment of chronic, stable CAD patients with a quantitative ischemic burden exceeding 10% of the myocardium.39-41 Furthermore, a decrease in ischemic burden by ≥5% with either PCI + OMT or OMT alone resulted in a significant increase in event-free survival over 5 years (Fig. 64–2). These data suggest that in select patients, a large myocardial ischemic burden on MPI testing may be a useful metric for the consideration of PCI as an initial treatment strategy. These subgroup analyses should be considered hypothesis generating and await prospective validation in larger numbers of patients with multivessel CAD.
| High risk (>3% annual mortality rate) |
| 1. Severe resting LV dysfunction (EF <35%) |
| 2. High-risk Duke Treadmill Score (score ≤ −11) |
| 3. Severe exercise-induced LV dysfunction (exercise EF <35%) |
| 4. Stress-induced large perfusion defect (particularly if anterior) |
| 5. Stress-induced multiple perfusion defects of moderate size |
| 6. Large fixed perfusion defect with LV dilation or increased lung uptake |
| 7. Stress-induced moderate perfusion defect with LV dilation or increased lung uptake |
| 8. Echocardiographic wall motion abnormality (involving >2 segments) developing at low dose of dobutamine (≤10 mg/kg/min) or at a low heart rate (<120 beats/min) |
| 9. Stress echocardiographic evidence of extensive ischemia |
| Intermediate risk (1%-3% annual mortality rate) |
| 1. Mild/moderate resting LV dysfunction (EF of 35%-49%) |
| 2. Intermediate-risk Duke Treadmill Score |
| 3. Stress-induced moderate perfusion defect without LV dilation or increased lung intake |
| 4. Limited stress echocardiographic ischemia with a wall motion abnormality only at higher doses of dobutamine involving ≤2 segments |
| Low risk (<1% annual mortality rate) |
| 1. Low-risk Duke Treadmill Score (score ≥5) |
| 2. Normal or small myocardial perfusion defect at rest or with stress |
| 3. Normal stress echocardiographic wall motion or no change of limited resting wall motion abnormalities during stress |
Figure 64–2.
The effect on event-free survival of decreasing ischemic myocardial burden ≥5% assessed by myocardial perfusion imaging in stable coronary artery disease patients enrolled in the Clinical Outcomes Utilizing Revascularization and Aggressive Drug Evaluation (COURAGE) trial. Patients were treated for 6 to 18 months with either optimal medical therapy (OMT) or OMT plus percutaneous coronary intervention. Reproduced with permission from Shaw et al.39
Coronary computed tomographic angiography (CCTA) provides an anatomic assessment of the epicardial coronary arteries. Because of its high spatial resolution and negative predictive value, CCTA is especially helpful to exclude important CAD in patients with a low pretest likelihood of disease. CCTA is also well suited to visualize suspected congenital coronary artery anomalies and great-vessel anatomy. Appropriate use criteria for the application of CCTA are under revision. Routine use of this technology in those with stable CAD is not recommended.42,43
Coronary artery calcification observed on EBCT or multidetector computed tomography strongly correlates with the presence of established atheroscerlosis.44 The coronary artery calcification score is a quantitative measure of overall vascular calcium burden. The presence of calcium in the vessel wall does not correlate with the degree of luminal obstruction, although the overall burden may predict future coronary heart disease events.45 Serial coronary artery calcification scoring to assess the rate of disease progression is not recommended.46,47
Referral for invasive coronary angiography in patients with chronic CAD is indicated for refractory symptoms or high-risk features on noninvasive testing. In individuals with symptoms suggestive but not diagnostic of CAD, invasive angiography may be appropriate if their occupations constitute a risk to themselves or others (eg, airline pilots, firefighters, others).48 In certain patients for whom the diagnosis of CAD on clinical grounds may be elusive due to atypical chest pain characteristics or asymptomatic ischemia (eg, diabetics), a lower threshold for coronary angiography may be appropriate. Due to atypical symptomatology and relatively lower diagnostic accuracy rates on conventional stress testing, women are sometimes considered for invasive angiography in the setting of an ambiguous presentation and/or inconsistent noninvasive data, but consideration of this approach should be weighed against the risks of the invasive procedure. Additional indications for invasive angiography include the evaluation of CAD in patients with reduced EF (<0.40), patients surviving SCD, or patients with ventricular arrhythmias and a high or intermediate likelihood of CAD.48 Table 64–6 lists the ACC/AHA recommendations for invasive angiography.
| Class I |
| 1. Patients with known or possible angina pectoris who have survived sudden cardiac death. (Level of Evidence: A) |
| Class IIa |
| 1. Patients with an uncertain diagnosis after noninvasive testing in whom the benefit of a more certain diagnosis outweighs the risk and cost of coronary angiography. (Level of Evidence: B) |
| 2. Patients who cannot undergo noninvasive testing due to disability, illness, or morbid obesity. (Level of Evidence: B) |
| 3. Patients with an occupational requirement for a definitive diagnosis. (Level of Evidence: B) |
| 4. Patients who by virtue of young age at onset of symptoms, noninvasive imaging, or other clinical parameters are suspected of having a nonatherosclerotic cause of myocardial ischemia (coronary artery anomaly, Kawasaki disease, primary coronary artery dissection, radiation-induced vasculoplasty). (Level of Evidence: B) |
| 5. Patients in whom coronary artery spasm is suspected and provocative testing may be necessary. (Level of Evidence: A) |
| 6. Patients with a high pretest probability of left main or three-vessel coronary artery disease. (Level of Evidence: A) |
| Class IIb |
| 1. Patients with recurrent hospitalization for chest pain in whom a definite diagnosis is judged necessary. (Level of Evidence: B) |
| 2. Patients with an overriding desire for a definitive diagnosis and a greater than low probability of coronary artery disease. (Level of Evidence: B) |
| Class III |
| 1. Patients with significant comorbidity in whom the risk of coronary angiography outweighs the benefits of the procedure. (Level of Evidence: C) |
| 2. Patients with an overriding personal desire for a definitive diagnosis and a low probability of coronary artery disease. (Level of Evidence: C) |