Surgical Treatment of Atrial Fibrillation
Sameh M. Said
Hartzell V. Schaff
BACKGROUND
Atrial fibrillation (AF) is by far the most common cardiac arrhythmia, and the prevalence increases with age, from 0.5% for patients in their 1950s to almost 9% among patients 80 years of age and older. It is estimated that approximately 2.3 million people in the United States have AF, and the incidence will continue to increase with increasing age in the population.
AF has three main serious sequelae: (1) patient anxiety due to rapid heart rate, (2) deterioration of cardiac hemodynamics related to loss of synchronous atrioventricular contraction, and (3) increased risk of thromboembolic complications and stroke. The basis of AF management is directed toward these complications and includes control of heart rate, prevention of thromboembolism, and, when possible, reestablishing and maintaining sinus rhythm. There is continued controversy regarding the efficacy of rhythm control versus rate control strategies. Unfortunately, long-term success of medical treatment of AF is poor. Five years after entry into the AFFIRM trial, only 63% of patients assigned to the rhythm control group were in sinus rhythm. Furthermore, chronic medical therapy has a number of unwanted side effects.
For these and other reasons, there has been considerable interest in novel strategies for control and treatment of AF over the last three decades. One of the earliest approaches to control AF was “ablate and pace strategy” with atrioventricular nodal ablation and permanent pacemaker implantation. Although atrioventricular node ablation controls the heart rate, the patient remains in AF, is pacemaker dependent, and still incurs the risks of thromboembolic stroke. Compared with patients with AF managed medically, patients who undergo atrioventricular node ablation and permanent transvenous pacing exhibit similar exercise performance and ventricular function. In patients with diminished left ventricular function, atrioventricular nodal ablation and permanent pacing result in a small but significant improvement in left ventricular function compared with medical therapy alone.
Through the work of Cox and associates, surgical and catheter techniques for ablating AF have been developed and are highly refined. Patients with AF who come to surgical attention fall into two general groups. First, are patients with isolated (lone) AF who are symptomatic despite medical treatment and/or attempts at catheter ablation. In the second, larger group, are patients with AF and associated structural heart disease that requires operative treatment. Selection of a surgical ablative method differs depending on the indication for operation, the disability caused by the arrhythmia, and the anticipated morbidity of the procedure for AF and any additional necessary surgery. For example, the standard “cut-and-sew” maze procedure may be the most effective and appropriate treatment for a highly symptomatic patient with isolated AF, but the technique would not be appropriate for an elderly patient undergoing double-valve replacement who has few symptoms from AF and whose heart rate is easily controlled medically.
SURGICAL ABLATION
The Cox-Maze Procedure
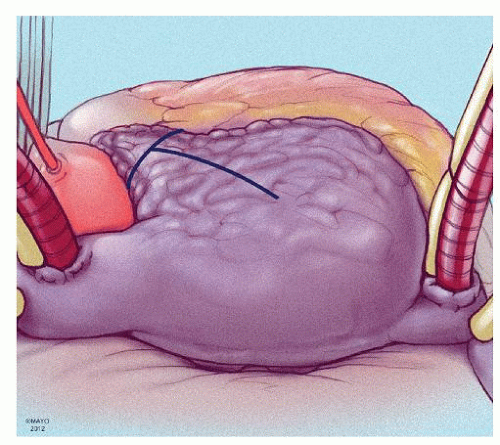
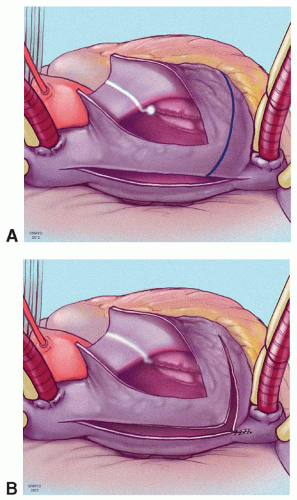
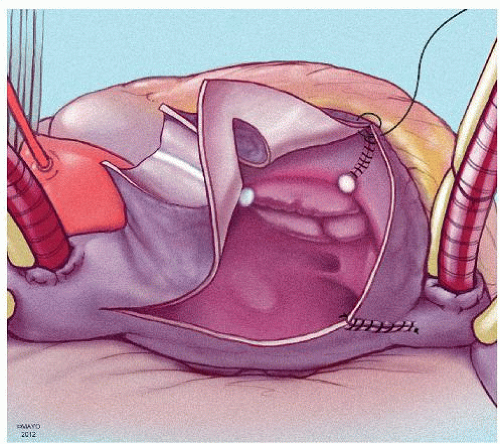
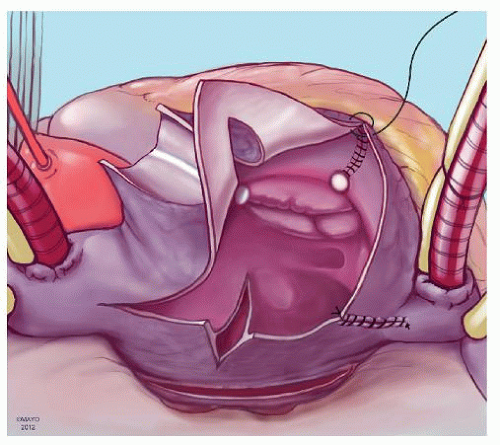
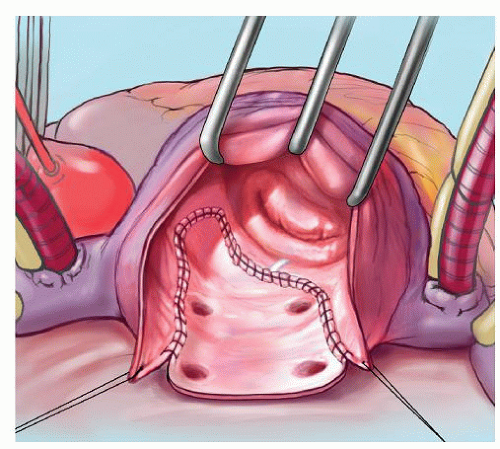
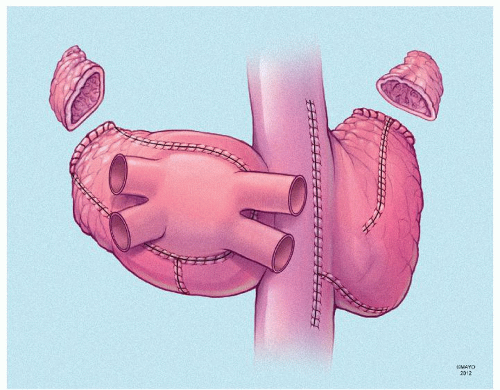
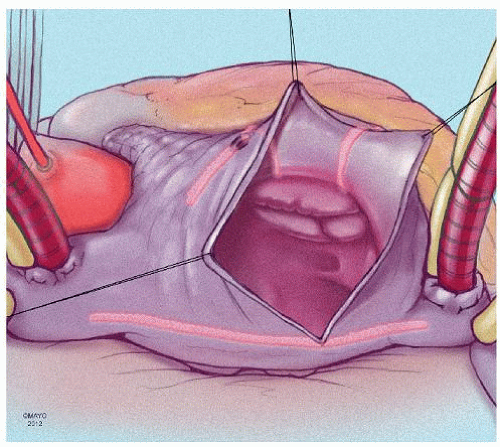
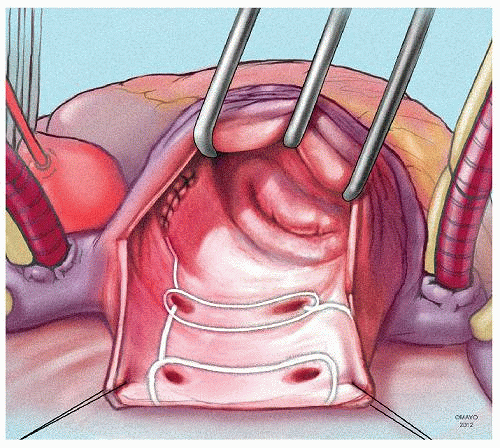
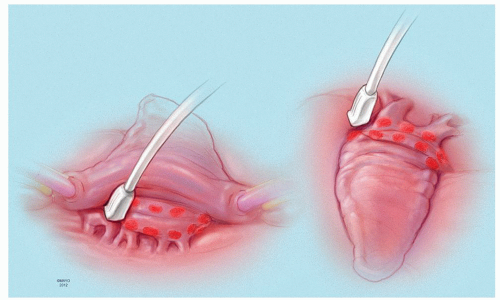
The prototype for all current surgical procedures for AF is the maze procedure developed by Cox and coworkers, and we consider the standard “cut-and-sew” maze procedure to be the most reliable and effective method in creating complete transmural atrial lesions for AF ablation. We continue to use the “cut-and-sew” maze (Figs. 65.1, 65.2, 65.3, 65.4, 65.5, 65.6) procedure in selected patients with only two modifications from the original description of the procedure. First, we have replaced the incision on the medial aspect of the right atrium from the cut edge of the right atrial appendage to the tricuspid valve annulus with a cryolesion; this avoids the division of the frequently seen branch of the right coronary artery that supplies the sinoatrial node and reduces the risk of postoperative sinus node dysfunction (Fig. 65.2A and 65.2B). The second modification involves the left atrium where we prefer to extend the incision that encircles the pulmonary veins to the orifice of the left atrial appendage, and then, after excising the appendage, close the orifice transversely as part of the encircling incision. Alternatively, cryolesions can be utilized as part of the lesion encircling the pulmonary veins when the exposure of this area is poor, and incisions may be difficult to close.
Many other surgical procedures have been proposed for the treatment of AF, and these operations utilize a variety of lesion sets and energy sources. Each of the modified procedures has potential advantages regarding ease of application and, in some cases, minimally invasive approaches. However, there is no conclusive evidence that any of the modified procedures yields better or even similar results to the original operation described by Cox.
Alternate Surgical Approaches for Atrial Fibrillation Ablation
Bilateral Pulmonary Vein Isolation
Pulmonary vein isolation (PVI) can be performed epicardially or endocardially
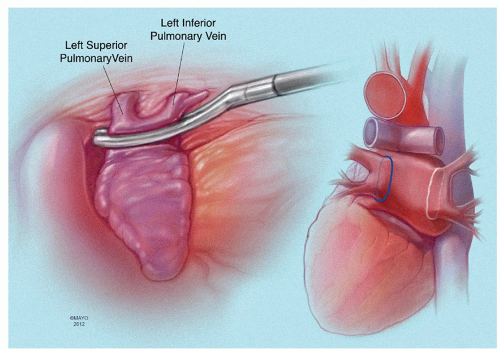
with or without left atrial appendage exclusion or excision. We view left atrial appendage ligation as an important step in surgical treatment of AF and never omit this. Epicardial PVI may be useful as an adjunctive procedure to operations that do not include left atriotomy (Fig. 65.7). When the left atrium is opened, as is the case with mitral valve repair or replacement, a bipolar radiofrequency (RF) clamp can be used to isolate the pulmonary veins by connecting the left atriotomy to the stump of the amputated left atrial appendage (“box lesion”).
with or without left atrial appendage exclusion or excision. We view left atrial appendage ligation as an important step in surgical treatment of AF and never omit this. Epicardial PVI may be useful as an adjunctive procedure to operations that do not include left atriotomy (Fig. 65.7). When the left atrium is opened, as is the case with mitral valve repair or replacement, a bipolar radiofrequency (RF) clamp can be used to isolate the pulmonary veins by connecting the left atriotomy to the stump of the amputated left atrial appendage (“box lesion”).
If the patient can be converted to sinus rhythm, it is important to check for exit block to confirm interruption of conduction from the pulmonary veins. However, demonstration of exit block or conduction delay acutely does not guarantee that lesions are transmural and that conduction will not reappear late after operation. Also, it is important to recognize that epicardial application of RF or cryoclamps to the atrial cuffs around the pulmonary vein orifices results in a double layer of atrial tissue to be ablated; with this method, transmurality may be difficult to achieve, especially in a beating perfused heart.
Minimaze Procedure
The term “minimaze” commonly refers to minimally invasive epicardial procedures without the use of cardiopulmonary bypass (Figs. 65.8 and 65.9). Lesion sets include a combination of pulmonary vein encircling lesion, left atrial isthmus lesion to the orifice of the left atrial appendage, and lateral left atrial incision without cryolesions. Wolf describes a minimally invasive procedure for AF performed through bilateral thoracoscopic ports. The operation includes bilateral antrum isolation and partial cardiac denervation. More recently, Sirak and associates describe the “five-box” thoracoscopic maze. In this procedure, bilateral thoracoscopy is performed with extensive dissection of the dome of the left atrium and transverse sinus. The procedure aims at compartmentalization of the posterior left atrium with ablation lines and connecting these lines to the mitral valve annulus, thus interrupting all the possible reentry circuits for AF.
Saline-Irrigated Cooled-Tip Radiofrequency Ablation
A “triangle-like” lesion set is created using a saline-irrigated, cooled-tip RF ablation. PVI is performed within the confines of the triangle, as well as left atrial appendage exclusion. One vertex of the triangle meets at the midportion of the posterior mitral valve annulus.
Alternate Energy Sources
The use of alternate energy sources has been increasing in the recent era due to the perceived technical complexity of the classic “cut-and-sew” Cox-maze. Cryoablation can be achieved using either nitrous oxide or argon cryoprobes, and the primary difference between the cooling techniques is the speed at which cooling of tissues occur. A purported advantage of cryoablation is the preservation of the cardiac fibrous skeleton. Cryoablation has been used extensively in clinical practice and appears quite effective in the arrested heart. However, in the beating heart with atria that are blood-filled and perfused, complete transmural lesions may be difficult or impossible to create. Additional cooling time is necessary because of warming of the tissue by the blood interface, and there is a theoretical risk of thrombus formation within the atrium.
RF energy employs alternate current to transfer energy to atrial tissue, and a variety of instruments have been developed including rigid unipolar probes with cooled tips, flexible unipolar probes, and bipolar clamps with and without irrigation. RF ablation can be applied to the epicardial or endocardial surfaces of the atrium in a unipolar configuration. Bipolar RF probes configured as clamps have the advantage of focused discrete lesions, thus, minimizing the potential risk of injury of adjacent structures. Saline irrigation of the tissue
during RF ablation has a cooling effect that prevents char accumulation and increases energy transfer to the tissues. Animal studies suggest a higher success rate of creating transmural lesions with irrigated delivery of RF compared with ablation without irrigation.
during RF ablation has a cooling effect that prevents char accumulation and increases energy transfer to the tissues. Animal studies suggest a higher success rate of creating transmural lesions with irrigated delivery of RF compared with ablation without irrigation.
Less commonly used alternate energy sources include microwave energy where transmission of electromagnetic waves ablate tissues through the heat produced; with this method, there has been concern about damage to adjacent structures including reported cases of coronary artery stenosis. With laser energy, the tissue is heated directly producing photocoagulation. The transmurality of the lesion as well as the concern for collateral damage are the limiting factors. High-intensity focused ultrasound is promising because of its ability to ablate at specific depth.
Left Atrial Ganglion Ablation
Ectopic impulses originating from the autonomic ganglia adjacent to the pulmonary veins have been implicated in initiating AF. These ganglia are found in the fatty epicardial tissue and have been recently mapped and ablated, which reduces the recurrence of medically refractory AF. The standard locations of these active ganglia have been previously described. The procedure is typically performed through median sternotomy and with the use of cardiopulmonary bypass. Intraoperative mapping is required with 12 V of high-frequency stimulation at a cycle length of 50 milliseconds and a pulse width of 1.5 milliseconds. An active ganglion is identified if stimulation results in a doubling of the R-R interval. All active sites were immediately ablated with an RF pen (Fig. 65.10).
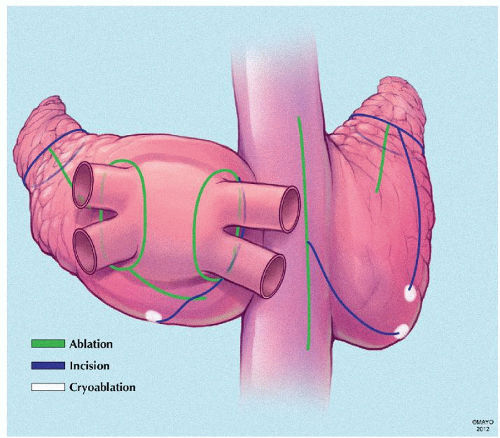
Cox-Maze IV (Ablation-Assisted Cox-Maze)
Introduced in 2002, the Cox-maze IV procedure is another modification to the standard “cut-and-sew” maze that serves to further simplify the technique. This is done by replacing most of the incisions with bipolar RF energy and using cryoenergy in addition to achieve the complete biatrial lesion sets. This has the potential advantage of shorter ablation times, can be performed in a less invasive manner and by localization of the ablation lines between clamps, there is minimal collateral damage. However, its durability has been questioned in comparison with the maze III procedure in terms of transmurality (Fig. 65.11).
Minimally Invasive Approaches
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree