Surgery for Complications of Myocardial Infarction
Philip W. Carrott
Timothy J. Gardner
Irving L. Kron
INTRODUCTION
Surgical intervention is often required to manage acute mechanical complications of myocardial infarctions. These mechanical complications, which are responsible for 15% to 20% of deaths after acute myocardial infarction, have historically been considered to include free ventricular wall rupture, acute ventricular septal defects (VSDs), and acute ischemic mitral regurgitation (MR). An acute myocardial infarction complication increasingly considered to be mechanical and amenable to surgical intervention is “pump failure,” or cardiogenic shock refractory to maximal medical treatment and revascularization. Surgical techniques used for this complication include ventricular assist device implantation and transplantation.
FREE VENTRICULAR WALL RUPTURE
Incidence and Pathogenesis
Free ventricular wall rupture is found in approximately one-fourth of patients who die within 3 weeks of an acute myocardial infarction. Overall, free wall rupture occurs in up to 11% of patients after acute infarction. Rupture location depends on the site of the infarct, with the rupture tract most commonly occurring between viable and necrotic myocardium. Rupture usually occurs after acute expansion of a transmural infarction. Infarct hemorrhage may play a role because revascularization with thrombolytic therapy but not angioplasty has been found to be independently associated with rupture of both the free wall and the septum.
Clinical Presentation and Diagnosis
Free wall rupture has acute, subacute, and chronic presentations. Acute rupture usually results in death within minutes. Subacute rupture represents 20% to 40% of the cases of free wall rupture and presents as cardiac tamponade progressing to shock. Echocardiography evaluating for a pericardial effusion in the presence of ventricular wall defects in a patient with tamponade physiology after acute infarction is used for diagnosis. Chronic rupture is rare and involves a contained ventricular leak that results in a pseudoaneurysm. Most patients have symptoms of congestive heart failure (CHF), chest pain, or dyspnea, although 12% to 23% are asymptomatic. More than two-thirds of patients have a murmur, and virtually all patients have nonspecific electrocardiogram (ECG) abnormalities. Most patients have cardiomegaly on chest X-ray. Angiography, echocardiography, computed tomography scans, radionuclide scans, and magnetic resonance imaging can be used to identify the pseudoaneurysm.
Natural History
Acute rupture rarely allows time for intervention and is invariably fatal. Patients with subacute rupture have a median survival of 8 hours after symptom onset, with a range from 45 minutes to 6.5 weeks. Only 17 cases of survival without surgery for subacute rupture have been reported. The natural history of chronic rupture is not well defined. Frances et al. identified 290 patients reported in the literature as having a left ventricular pseudoaneurysm, 139 of which had resulted from a myocardial infarction. Of 31 patients treated conservatively, 15 died at <1 week from infarction and the other 16 survived long term, which suggests that chronic pseudoaneurysms are relatively stable.
Preoperative Treatment and Operative Timing
Acute rupture is generally not amenable to any attempts at repair. Emergent operative repair is needed for any patient with a subacute rupture, and the patient must be brought immediately to the operating room. Pericardiocentesis at the time of echocardiography can provide short-term hemodynamic improvement in some patients, allowing some degree of stability while preparing for surgery. Inotropic agents, fluid infusion, vasoconstrictors, and an intra-aortic balloon pump (IABP) can also be used to maintain hemodynamics during transfer to the operating room.
Timing of surgical repair for chronic rupture depends on the interval between infarction and diagnosis. Patients who are within a few months of infarction are thought to have a high risk for rupture and should undergo urgent cardiac catheterization to evaluate the extent of coronary artery disease followed by operative repair of the pseudoaneurysm. The need for surgery in patients who present after an extended period after infarction depends on whether the pseudoaneurysm is >3 cm, expanding, or symptomatic, or if the patient has MR and/or severe coronary artery disease that requires intervention.
Operative Technique
Patients with subacute rupture are rapidly prepared for a standard median sternotomy, with close monitoring of hemodynamics during anesthesia induction because significant hypotension can occur. Close hemodynamic monitoring is also required when the pericardium is decompressed because hypertension can occur and possibly worsen the rupture size and the amount of bleeding. The tear location and status and the patient status determine the need for cardiopulmonary bypass. Cardiopulmonary bypass is instituted in all hemodynamically unstable patients. Bypass is also used if the rupture cannot be exposed adequately without excessive compromise of circulation or if the bleeding from the rupture cannot be controlled or prevent adequate repair.
Subacute rupture can be repaired by various techniques, which should be kept as simple as possible. Sutures should not be placed into friable myocardium. Epicardial repair can be performed by the placement of a patch of pericardium, Dacron, or polytetrafluoroethylene felt over the ventricular defect; the patch is sutured to healthy myocardium along the periphery of the infarct (Fig. 56.1A). The rupture can also be closed directly with horizontal mattress sutures buttressed with Teflon felt strips and a patch secured over the repair (Fig. 56.1B). Biocompatible glue can also be used to further reinforce the patch over the defect (Fig. 56.1C). Simple epicardial repair is not appropriate when an intraventricular defect such as septal rupture or papillary muscle rupture also exists. Operative options in these cases require cardiopulmonary bypass and aortic cross-clamping with infarct excision and defect closure using a patch or infarct exclusion, which are described in detail in the following sections.
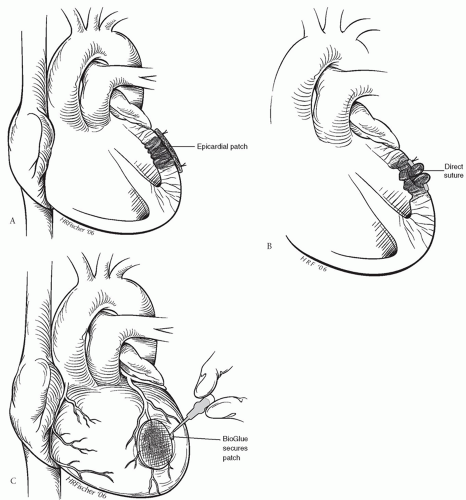 Fig. 56.1. Repair of left ventricular free wall rupture with (A) epicardial patching, (B) direct suture, and (C) application of patch secured to ventricle with BioGlue. |
A new product, Tachosil (Takeda Pharmaceuticals International GmbH, Zurich, Switzerland), which is a white collagen patch with a fibrinogen and thrombin coating on one side, has been used to buttress free wall rupture in leaking and blown out myocardium without need for further operative patching. The technique was used in six patients with good operative and long-term outcomes. For selected patients with small defects or oozing rupture, this simple repair offers a good option with minimal operative trauma to the myocardium.
The decision to repair a chronic ventricular rupture is partially based on the presence of either mitral dysfunction or coronary disease. Cardiopulmonary bypass is instituted, and the chronic rupture site is repaired similar to an acute rupture. Anterior pseudoaneurysms can usually be closed directly because of the presence of fibrotic edges. Posterior pseudoaneurysms are closed with a patch so ventricular geometry is not distorted. Even for cases in which the primary purpose of the operation is to repair a large or expanding pseudoaneurysm, cardiopulmonary bypass is indicated to allow adequate exposure for repair and to also prevent the systemic embolization of thrombotic material from within the pseudoaneurysm cavity.
Survival
Most reports of surgical intervention for subacute rupture involve a limited number of patients. These reports, however, indicate that a significant number of patients can be saved from this lethal condition with prompt diagnosis and intervention. In one series, two of five patients who were extremely unstable and underwent emergent repair of a subacute rupture survived long term. In another series of five patients treated similarly, there were four survivors. In a series of six patients treated with a sutureless patch over an 8-year period, five patients survived to leave the hospital. The experience with surgical management of chronic rupture is also limited and largely anecdotal. In one series, 8 of 12 patients treated for a chronic rupture were long-term survivors. All deaths occurred in patients with poor ventricular function or mitral valve disease.
VENTRICULAR SEPTAL DEFECT
Incidence and Pathogenesis
An acute VSD is an uncommon but lethal complication of infarction. A VSD that occurs within 4 to 6 weeks of infarction is considered acute. Even though the septum is involved in up to 70% of all infarcts, acute VSD historically has complicated only 1% to 2% of all infarctions, with recent evidence suggesting that the incidence is now <1%. This decreased incidence may reflect the success of interventions that limit muscle necrosis and prevent transmural infarctions. However, the time between infarction and the VSD occurrence appears to be becoming shorter. Because infarct hemorrhage may play a role in septal rupture, thrombolysis may accelerate the time course of VSDs in those patients in which they occur.
Patients with acute postinfarction VSD have an average age of 62.5 years; the maleto-female ratio is 3:2, although the incidence may be rising in women. Acute VSD usually occurs after occlusion of a coronary artery that results in a full-thickness infarction that is on average larger (25% of the total left ventricular wall mass) than an infarction that is not complicated by a VSD (15%). The infarcts in patients who develop a postinfarction VSD more commonly involve both ventricles than the infarcts in those patients who do not develop a VSD. The typical patient has single-vessel disease with poor collateral flow and presents with an anterior infarct. Although this complication may occur as early as a few hours and as late as a few weeks after infarction, the diagnosis is most commonly made within 4 days of the onset of infarction symptoms. The time of septal rupture correlates with the period when necrotic muscle from the infarction has not been adequately replaced by fibrous connective tissue. Defect size ranges from 0.3 to 4.0 cm, with an average of 1.7 cm.
Historically, acute septal defects were described primarily in the anteroapical septum as a result of left anterior descending artery occlusion. Recent data, however, suggest that the proportion of posterior VSDs is rising, and these now represent one-third to one-half of VSDs. Posterior VSDs result from occlusion of a dominant right coronary artery or a dominant circumflex artery. Defects are classified as either simple or complex. Simple defects are usually located anteriorly. Complex defects are usually located inferiorly and have a worse prognosis. Multiple VSDs occur in 5% to 11% of cases. One-third of cases involve mitral valve regurgitation, which results from either papillary muscle infarction (15% of cases) or left ventricular dysfunction and mitral annular dilation. The MR seen due to left ventricular dysfunction generally resolves with repair of the VSD, whereas papillary muscle rupture requires valve replacement.
Clinical Presentation and Diagnosis
An acute VSD typically presents with recurrent chest pain, a new holosystolic murmur and palpable left sternal thrill, and hemodynamic deterioration a few days after acute infarction. Septal rupture causes left-to-right shunting that can result in heart failure. Clinical presentation varies from an asymptomatic murmur to cardiogenic shock. Symptoms occur due to both ventricular dysfunction and the shunting caused by the VSD. Failure occurring with an anterior VSD is usually the result of both the VSD and extensive left ventricular infarction, whereas failure occurring with a posterior VSD is usually due to extensive right ventricular infarction. Infarct location on the ECG correlates highly with VSD location. The ECG often shows a rightward QRS axis shift and a right-bundle-branch block, and approximately one-third of patients get a transient atrioventricular conduction block before septal rupture.
The clinical appearance of an acute VSD is very similar to that of acute MR. Physical examination and the ECG are used to distinguish between the two entities. The murmur associated with a VSD is most prominent at the left sternal border and is often accompanied by a thrill, whereas the murmur from acute MR is best heard at the apex and has no thrill. In addition, acute MR is more commonly associated with inferior infarctions and no conduction abnormalities. Echocardiography has a very high sensitivity and specificity in identifying the presence, size, and location of a VSD and shows a diagnostic trans-septal flow jet, as well as an echo-free area of the septum.
Demonstrating a 9% step-up in oxygen saturation from the right atrium to the pulmonary artery during right heart catheterization with pulmonary artery placement also confirms shunt presence due to VSD.
Natural History
Patients with a postinfarction VSD treated medically have a 1-year mortality as high as 97%. One-fourth of patients die within 24 hours, and 80% die within 4 weeks. Death usually occurs as a result of end-organ failure due to shock.
Preoperative Therapy and Operative Timing
Because early surgical repair of acute VSDs had a very high mortality, surgical repair was previously delayed for 4 to 6 weeks to allow hemodynamic stabilization and fibrosis of the infarct to facilitate suturing around the VSD. Although some patients survived this delayed management, it became clear that only low-risk patients survived to surgical repair. Surgical intervention is now recognized as required before cardiogenic shock results in irreversible end-organ damage, and operative timing for repair is dictated by the patient’s hemodynamic status. Completely stable patients, comprising only a
small proportion of patients, should have elective repair sometime during their hospitalization for the acute infarction.
small proportion of patients, should have elective repair sometime during their hospitalization for the acute infarction.
Patients requiring pharmacologic support for heart failure require intervention within 12 to 24 hours of diagnosis. A patient in cardiogenic shock is a true surgical emergency and needs immediate repair. Patients who have already developed multisystem failure or sepsis are extremely at high risk for emergency surgery and likely require further attempts at stabilization, with antibiotics as needed, before attempted repair. As more experience with ventricular assist devices evolves, their use in this complex population as a temporizing measure is expanding. The Impella 5.0 catheter (Abiomed, Inc., Danvers, MA) is most useful since it can be placed through a femoral artery cut-down and does not require opening the chest for cannulation. As the name implies, the maximum flow is 5 l/min, which should help to off-load the right ventricle and allow the myocardium to heal. A delayed repair may then be undertaken with stronger myocardial tissue. Case reports describe deferring operative repair for around 14 days with a mortality rate of 40%.
The preoperative treatment goal is to divert blood systemically and maintain cardiac output, blood pressure, and coronary flow. Diuretics, inotropes, and vasodilators can minimize the left-to-right shunt; however, vasodilators are often not tolerated due to systemic hypotension. Vasoconstrictors increase afterload and can worsen left-to-right shunting and should be avoided if possible. An IABP may improve cardiac output by reducing afterload and decreasing shunting and is mandatory in management of VSD patients who present in shock. However, IABP use has peak improvement within 24 hours and then no further benefit, so surgery should not be delayed beyond this time.
The role of preoperative coronary angiography is not completely clear; delaying surgical repair to perform cardiac catheterization during a period of patient instability is risky. In addition, the use of coronary angiography and coronary artery bypass grafting did not show improved short-term or long-term survival in a study of 179 patients with postinfarction VSD. However, routine coronary angiography detected significant coronary artery disease beyond the vessel that resulted in the acute infarction in 28 patients in another study of 54 patients with acute postinfarction VSDs. These patients underwent concomitant revascularization and experienced similar early and late survival compared with patients without associated coronary disease, suggesting that revascularization controlled the added risk of associated coronary disease. In another recent multi-institutional review, 42 of 65 patients who had coronary bypass grafting at the same time as VSD repair had significantly better survival both in the short-term and at 4 years. Given that coronary angiography can be performed very rapidly, a reasonable policy is to perform angiography with a limited amount of contrast in all patients who can be temporarily stabilized. Patients who are in severe shock should proceed directly to surgery for VSD repair.
Operative Technique
Several techniques can be used to repair acute postinfarction VSDs. Regardless of the specific technique utilized, a number of technical guidelines have been developed. A median sternotomy is performed with expeditious establishment of cardiopulmonary bypass using bicaval venous drainage. Cardioplegia should be first administered antegrade followed by retrograde via the coronary sinus to achieve optimal myocardial protection. In addition, patients with critical coronary stenoses identified preoperatively should undergo revascularization prior to VSD repair in order to maximize myocardial protection. A transinfarct approach to the VSD should be used. Because surgical success is completely dependent on adequate VSD closure, thorough evaluation for multiple defects must be performed both preoperatively and intraoperatively. In patients with associated significant MR, mitral valve replacement is needed only if frank papillary muscle rupture has occurred. The septal defect and the infarctectomy must be closed without tension, which generally necessitates the use of prosthetic material. Finally, all suture lines must be buttressed with pledgets or Teflon felt.
Apical septal ruptures can be repaired using the technique of apical amputation described by Daggett (Fig. 56.2). An incision through the infarct is made, and all necrotic muscle of both ventricles and the septum is debrided. The remaining apical parts of the ventricles and septum are reapproximated by a row of interrupted mattress sutures using buttressing strips of felt.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


