Epidemiology
Coronary artery disease is the leading cause of morbidity and mortality in Western society and is a worldwide epidemic. In 2004, it was estimated that worldwide, ischemic heart disease was responsible for 9.4% of all deaths (2.5 million) in low-income countries and 16.3% (1.3 million) of all deaths in high-income countries.1 Approximately 935,000 Americans suffer from an acute myocardial infarction (AMI) per year, one-third of which are caused by an acute ST-segment elevation myocardial infarction (STEMI).2 The incidence of AMI has declined over the past 2 decades from 244 per 100,000 population in 1975 to 162 per 100,000 population in 2006.2 The in-hospital mortality rate also has declined from 18% in 1975 to 10% in 2006.3 Despite these improvements, AMI continues to be a major public health problem, and it has been estimated that the number of years of life lost because of an AMI is 15 years, and the cost to American society (both direct and indirect) is $165.4 billion per year.2
The management of STEMI patients is complex, multidisciplinary, and involves the following four different stages of care: (1) prehospital care, (2) emergency department, (3) cardiac catheterization laboratory, and (4) coronary care unit. This chapter discusses the diagnosis and management of STEMI patients in each of these four settings. The pathophysiology of disease is discussed in Chap. 57 and the acute coronary syndromes of unstable angina and non–ST-segment elevation myocardial infarction are discussed in Chap. 59.
Diagnosis
The classic symptom of acute myocardial ischemia is precordial or retrosternal discomfort, commonly described as a pressure, crushing, aching, or burning sensation. Radiation of the discomfort to the neck, back, or arms frequently occurs, and the pain is usually persistent rather than fleeting. The discomfort typically achieves maximum intensity over several minutes and can be associated with shortness of breathe, nausea, diaphoresis, generalized weakness, and a fear of impending death. Some patients, particular the elderly, may also present with syncope, unexplained nausea and vomiting, acute confusion, agitation, or palpitations. Symptoms in the advanced elderly (>75 years old) are more likely to be atypical than in younger patients and can lead to a missed diagnosis if a medical professional is not vigilant in the initial assessment.
Approximately 20% of AMI patients are asymptomatic or have atypical symptoms that are not initially recognized. Painless myocardial infarction occurs more frequently in the elderly, women, diabetics, and postoperative patients. These patients tend to present with dyspnea or frank congestive heart failure as their initial symptom.4
Patients often appear anxious and uncomfortable. Those with substantial left ventricular (LV) dysfunction at presentation may have tachypnea, tachycardia, pulmonary rales, and a third heart sound. The presence of a systolic murmur suggests ischemic dysfunction of the mitral valve or ventricular septal rupture.
In patients with right ventricular (RV) infarction, increased jugular venous pressure, Kussmaul sign (rise in jugular venous pressure with inspiration), and an RV third sound may be present. Such patients typically have inferior infarctions due to proximal right coronary artery occlusion, usually without evidence of left-heart failure, and may have exquisite blood pressure sensitivity to nitrates or hypovolemia. In patients with extensive LV dysfunction, shock is indicated by hypotension, diaphoresis, cool skin and extremities, pallor, oliguria, and possible altered mental status.
Electrocardiogram
The classic initial electrocardiographic (ECG) manifestations of STEMI are discussed in Chap. 15 and involve an increase in the amplitude of the T wave (peaking), followed within minutes by ST-segment elevation. The R wave may initially increase in height but soon decreases, and often Q waves develop. If the jeopardized myocardium is reperfused, the ST segment may promptly decrease, although T waves can remain inverted, and Q waves may or may not regress. Persistent ST-segment elevation after restoration of flow in the epicardial coronary artery is a marker of abnormal myocardial perfusion and associated with an adverse prognosis. In the absence of reperfusion, the ST segment gradually returns to baseline in several hours to days, and T waves become symmetrically inverted. Failure of the T wave to invert within 24 to 48 hours suggests regional pericarditis.
The specific leads with ST-segment elevation can help localize the infarct (see Chap. 15): ST-segment elevation in the inferior, anterior, or high lateral leads is seen with infarction of the corresponding areas of myocardium. ST-segment elevation in lead aVR is more frequent in patients with left main artery occlusion than in patients with left anterior descending coronary artery or right coronary artery occlusion.5 In a study of STEMI patients, ST-segment elevation in lead aVR that was greater than or equal to the extent of ST-segment elevation in lead V1 had 81% accuracy for diagnosing left main occlusion.5 ST-segment elevation in lead V1, in the setting of inferior myocardial infarction suggests RV involvement. Because no leads on the standard 12-lead ECG directly represent the posterior myocardium, isolated infarction of this area may be difficult to diagnose but is typically manifested by ST-segment depression in V1-V3, a mirror image of anterior myocardial infarct.6 Depending on the affected artery (right coronary vs circumflex), posterior myocardial infarction is frequently associated with either inferior or lateral injury with ECG manifestations of STEMI corresponding to those areas of myocardium. Sensitivity of detecting posterior myocardial infarction may be enhanced by the use of additional ECG leads (V7-V9) designed to represent the posterolateral myocardium.7,8 ST-segment elevation in these additional leads is suggestive of posterior involvement.
New-onset left bundle-branch block (LBBB) in the setting of chest pain is typically considered and treated as an STEMI. The diagnosis of STEMI in the setting of old LBBB can be difficult. Findings suggesting STEMI include (1) a pathologic Q wave in leads I, aVL, V5, or V6 (2 leads); (2) precordial R-wave regression; (3) late notching of the S wave in V1 to V4; and (4) deviation of the ST segment in the same direction as that of the major QRS deflection.9 Similar findings may be expected in RV pacing with LBBB structure of the QRS. In one study, patients with paced rhythm had higher in-hospital and 1-year mortality, in part because of undertreatment, which illustrates the difficulty of AMI diagnosis in this setting.10
Analysis of ECG data from the Global Use of Strategies to Open Occluded Coronary Arteries (GUSTO) I study identified three criteria for diagnosing myocardial infarction in the presence of the LBBB: (1) ST-segment elevation ≥1 mm concordant with the QRS complex; (2) ST-segment depression ≥1 mm in leads V1, V2, or V3; and (3) ST-segment elevation ≥5 mm discordant with the QRS.11
The ECG, however, has several limitations: (1) Even though ST-segment elevation usually signifies acute coronary occlusion, acute coronary occlusion may not cause ST-segment elevation in certain circumstances, such as a circumflex artery occlusion; (2) in addition, not all ST-segment elevation is caused by AMI; and (3) finally, an old LBBB limits the usefulness of the ECG for AMI diagnosis in this subset of patients.
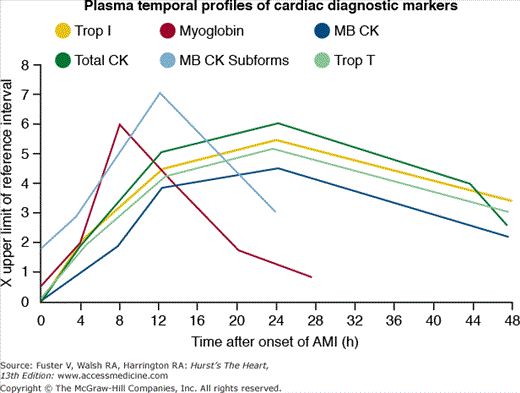
Damaged cardiomyocytes release several proteins in the circulation, including myoglobin, creatine kinase (CK) and its myocardial band isoenzyme (CK-MB), troponins (I and T), myoglobin, aspartate aminotransferase, and lactate dehydrogenase (see Chap. 58). Figure 60–1 shows the timing of release.12 Cardiac troponins are currently the preferred biomarkers for myocardial damage because of their high sensitivity and specificity.13 CK-MB is the best alternative, if cardiac troponin assays are not available. CK-MB, because of its more rapid appearance and disappearance from the blood, can be used (1) in patients presenting early after symptom onset; (2) to time the onset of injury if the troponin is increased; and (3) to detect reinfarction later in the hospital course. Determinations of total CK, aspartate aminotransferase, and lactate dehydrogenase are no longer recommended. Blood sampling for biomarker determination is recommended at hospital admission, at 6 to 9 hours, and at 12 to 24 hours if the earlier samples were negative and the clinical index of suspicion is high (see Chap. 58).13
Figure 60–1.
Shown here is the temporal profile of the diagnostic biomarkers used for detecting acute myocardial infarction (AMI). The plasma temporal profile for early detection is illustrated for myoglobin and myocardial band (MB) creatinine kinase (CK) subforms. The markers MB CK, total CK, and cardiac troponins (Trop) I and T are all released with a similar initial time profile. However, troponins I and T remain elevated for 10 to 14 days and thus are better markers for late diagnosis than that of MB CK.
Myoglobin is a 17.8-kDa protein that is released from injured myocardial cells. As shown in Fig. 60–1, myoglobin release occurs within hours after the onset on infarction, reaches peak levels at 1 to 4 hours, and remains elevated for approximately 24 hours. Although the rapid rise allows for its use as an early marker for STEMI, myoglobin is not specific to myocardial cells and should not be used in isolation as a method for diagnosing myocardial infarction.
The MB isoenzyme of creatine kinase is present in largest concentration in myocardium, although small amounts (1%-2%) can be found in skeletal muscle, tongue, small intestine, and diaphragm. CK-MB appears in serum within approximately 3 hours after the onset of infarction, reaches peak levels at 12 to 24 hours, and has a mean duration of activity of 1 to 3 days.12 Other cardiac, but non-AMI etiologies of increased CK-MB levels can occur after cardioversion, cardiac surgery, myopericarditis, percutaneous coronary intervention (PCI), and occasionally after rapid tachycardia. Noncardiac causes of increased CK-MB levels may occur with hypothyroidism, extensive skeletal muscle trauma, rhabdomyolysis, the muscular dystrophies, and some other neuromuscular disorders.
Occasionally, the concentration of CK-MB isoenzyme may be increased in the presence of normal total levels of CK enzyme. This finding usually indicates a small amount of myocardial necrosis in a patient whose baseline total CK enzyme level is at the low-normal end of the range (see Chap. 58).
The cardiac troponins regulate the interaction of actin and myosin and are more cardiac-specific than CK-MB. There are two isoforms of cardiac troponin: T and I. Their levels start to rise 3 to 12 hours after the onset of ischemia, peak at 12 to 24 hours, and may remain elevated for 8 to 21 days (troponin T) or 7 to 14 days (troponin I). Several new high-sensitivity troponin assays can detect lower concentrations of circulating troponins compared with previous assays. These assays were recently evaluated by two large multicenter studies revealing corroborating results. The high-sensitivity assays proved more sensitive in the diagnosis of myocardial infarction than older assays (94%-96% vs 85%-90%) at the expense of a reduced specificity (90.2% vs 97.2%).14,15 Due to lack of data clarifying the effect of the new high-sensitivity assays on clinical outcomes, the extent to which these assays may influence management and diagnosis of STEMI is currently uncertain. Nevertheless, it is clear that elevated troponin levels correlate with pathologically proven myocardial necrosis and indicate poor prognosis in patients with suspected acute coronary syndromes (see Chap. 56).16-20
Management in the Emergency Department
Rapid diagnosis is a pivotal component of the management of STEMI patients. Early diagnosis can be achieved with a prehospital ECG that is obtained in the field by emergency medical personnel and transmitted to an emergency department physician or cardiologist on-call. This allows for early administration of fibrinolytic therapy or activation of the cardiac catheterization team before arrival of the patient and has been demonstrated in several studies to reduce both the door-to-needle and door-to-balloon time.21-23 Although the American College of Cardiology (ACC)/American Heart Association (AHA) guidelines for STEMI considers the prehospital electrocardiogram as a class IIa indication,24 analysis of data from the National Registry of Myocardial Infarction revealed that only 4.5% (1599 of 35,370) of patients receiving thrombolytic therapy and 8% (1696 of 21,277) of patients undergoing primary PCI had a prehospital electrocardiogram.22 The analysis also showed that a prehospital ECG resulted in a significantly shorter door-to-needle time (24.6 vs 34.7 minutes; P < .0001) for those receiving fibrinolytic therapy and a shorter door-to-balloon time (94 vs 110 minutes; P < .0001) for primary PCI patients.22
The use of newer automated wireless technologies for the transmission of pre-hospital ECGs was studied in the ST-Segment Analysis Using Wireless Technology in Acute Myocardial Infarction (STAT-MI) Trial.25 In this study, the ECGs of 20 patients with STEMI were transmitted via cellular phones to a tertiary care center. Compared with data obtained in patients who did not receive prehospital ECGs, wireless transmission resulted in the improvement of the following times: door-to-intervention (80.1 vs 145.6 minutes; P < .001), door-to-cardiologist notification (14.6 vs 61.4 minutes; P < .001), door-to-arterial access (47.6 vs 108.1 minutes; P < .001), and time to first angiographic injection (52.8 vs 119.2 minutes; P < .001). These data further illustrate the importance of early evaluation and triage of patients with suspected STEMI to facilitate rapid and appropriate treatment. The availability and cost of this technology, however, may preclude its widespread use.
The cornerstone of STEMI therapy is a rapid and accurate evaluation at first medical contact. All patients presenting with complaints of chest discomfort should be rapidly triaged and allowed to bypass the emergency department waiting room. An ECG should be obtained within the first 10 minutes of arrival at the emergency department, and a focused history and physical examination assessing the symptoms and signs described in the diagnosis section of this chapter should be quickly performed.
The physical examination also provides a method for the risk stratification of STEMI patients. As shown in Table 60–1, the Killip classification can be used as a method to stratify patients and predict clinical outcomes.26 With modern therapy, the mortality of those in cardiogenic shock has improved from 83% to approximately 50%.
| Killip Class | Hospital Mortality (%) |
|---|---|
| I No congestive heart failure | 6 |
| II Mild congestive heart failure, rales, S3, congestion on chest radiograph | 17 |
| III Pulmonary edema | 38 |
| IV Cardiogenic shock | 81a |
Class I
The targeted history of STEMI patients taken in the emergency department should ascertain whether the patient has had prior episodes of myocardial ischemia, such as stable or unstable angina, myocardial infarction, coronary bypass surgery, or PCI. Evaluation of the patient’s complaints should focus on chest discomfort, associated symptoms, sex- and age-related differences in presentation, hypertension, diabetes mellitus, possibility of aortic dissection, risk of bleeding, and clinical cerebrovascular disease (amaurosis fugax, face/limb weakness or clumsiness, face/limb numbness or sensory loss, ataxia, or vertigo). (Level of Evidence: C)
A physical examination should be performed to aid in the diagnosis and assessment of the extent, location, and presence of complications of STEMI. (Level of Evidence: C)
A brief, focused, and limited neurologic examination to look for evidence of prior stroke or cognitive deficits should be performed on STEMI patients before administration of fibrinolytic therapy. (Level of Evidence: C)
A 12-lead ECG should be performed and shown to an experienced emergency physician within 10 minutes of emergency department arrival on all patients with chest discomfort (or anginal equivalent) or other symptoms suggestive of STEMI. (Level of Evidence: C)
If the initial ECG is not diagnostic of STEMI but the patient remains symptomatic and there is a high clinical suspicion for STEMI, serial ECGs at 5- to 10-minute intervals or continuous 12-lead ST-segment monitoring should be performed to detect the potential development of ST elevation. (Level of Evidence: C)
In patients with inferior STEMI, right-sided ECG leads should be obtained to screen for ST elevation suggestive of RV infarction. (Level of Evidence: B)
The initial management of patients in the emergency department includes the use of oxygen, antiplatelet therapy, β-blockers, analgesia, nitroglycerin, and anticoagulation.
Low-flow oxygen therapy delivered by nasal cannula should be routinely given during the first 24 to 48 hours and perhaps several days after acute myocardial infarction in most patients. Although routine use of supplemental oxygen is common practice, hard evidence supporting its use is lacking. Mild hypoxemia is not uncommon, even in the absence of apparent pulmonary congestion. Additionally, some patients may have dyspnea related to acute changes in LV compliance and secondarily increased pulmonary interstitial fluid. The Clinical Practice Guidelines recommend the following:24
Class I
Supplemental oxygen should be administered to patients with arterial oxygen desaturation (SaO2 less than 90%). (Level of Evidence: B)
Class IIa
It is reasonable to administer supplemental oxygen to all patients with uncomplicated STEMI during the first 6 hours. (Level of Evidence: C)
Aspirin decreases mortality in myocardial infarction and should be administered as early as possible and continued indefinitely in patients with acute coronary syndromes. In cases of aspirin allergy or major intolerance, clopidogrel should be substituted. In the International Study of Infarct Survival 2 (ISIS-2), 77,187 STEMI patients were randomized to treatment with aspirin, streptokinase, or placebo. The 5-week incidence of vascular death decreased by 23% with aspirin and heparin, by 25% with streptokinase and heparin, and by 41% with the combination of all three agents.28 Thus the effect of aspirin was surprisingly nearly as great as that of streptokinase alone in that study, and the benefits of each were partially additive. Thus 160 to 325 mg of chewable aspirin should be given to patients at presentation, with a subsequent dose of 75 to 325 mg daily. For those with a history of a documented significant adverse reaction to aspirin, 300 mg of clopidogrel can be used as an alternative. The Clinical Practice Guidelines recommend the following:24
Class I
Aspirin should be chewed by patients who have not taken aspirin before presentation with STEMI. The initial dose should be 162 mg (Level of Evidence: A) to 325 mg (Level of Evidence: C). Although some trials have used enteric-coated aspirin for initial dosing, more rapid buccal absorption occurs with non–enteric-coated aspirin formulations.
Thienopyridines are oral antiplatelet agents that inhibit platelet activation through the ADP-mediated pathway. Though several factors contribute to choice of thienopyridine (clopidogrel or prasugrel), as well as the initial dose, unless contraindicated, thienopyridines should be initiated upon presentation of STEMI. See “Adjuvant Antiplatelet Therapy” in this chapter for further discussion and Clinical Practice Guidelines regarding thienopyridines.
The results of clinical trials investigating the use of β-blockers in acute coronary syndromes have documented a decrease in early and late mortality. In pooled data from 28 trials of β-blockers, the average mortality decrease was 28% at 1 week, with the majority of benefit occurring in the first 48 hours.29 Specifically, reinfarction was reduced by 18% and cardiac arrest by 15%.29 The long-term effects of β-blockade for the secondary prevention of death after myocardial infarction were established by large-scale randomized trials. In a recent meta-analysis of 82 randomized trials of β-blockers, there was a significant reduction in long-term, but not short-term, mortality.30 The number of patients needed to treat with a β-blocker to prevent 1 death over 2 years was 42, compared with 24 for thrombolytics, 94 for standard-dose statins, and 153 for antiplatelet agents.
Traditionally, metoprolol has been the agent of choice and was initially administered intravenously followed by an oral dose. The Thrombolysis in Myocardial Infarction (TIMI) 2B study of early (immediate intravenous load followed by oral dosing) versus delayed (no intravenous load) therapy in STEMI patients who were undergoing reperfusion therapy with thrombolytic therapy showed no difference between the two groups in mortality or ejection fraction at the time of discharge.31 Data from COMMIT (Clopidogrel and Metoprolol in Myocardial Infarction Trial), which involved 45,852 patients, demonstrated that β-blockade resulted in a 15% to 20% relative risk reduction in the rate of recurrent infarction, but in a 30% relative increase in the risk of cardiogenic shock.32 Therefore, the use of β-blockade in patients with evidence of hemodynamic instability or heart block should be delayed until the patients become stable. Also, the routine initial intravenous administration of β-blockers is no longer considered standard therapy due to the potential risk of worsening status in patients with severe heart failure or cardiogenic shock. Finally, the benefits of β-blockade in patients undergoing primary PCI remains unclear, and there have been no randomized prospective studies. The Clinical Practice Guidelines recommend the following:24,33
Class I
Oral β blocker therapy should be initiated in the first 24 hours for patients who do not have any of the following: (1) signs of heart failure, (2) evidence of a low output state, (3) increased risk for cardiogenic shock, or (4) other relative contraindications to β blockade (PR interval greater than 0.24 seconds, second- or third-degree heart block, active asthma or reactive airway disease). (Level of Evidence: B)
Class IIa
It is reasonable to administer an IV β blocker at the time of presentation to STEMI patients who are hypertensive and who do not have any of the following: (1) signs of heart failure, (2) evidence of low output state, (3) increased risk for cardiogenic shock, or (4) other relative contraindications to β blockade (PR interval greater than 0.24 seconds, second- or third-degree heart block, active asthma, or reactive airway disease). (Level of Evidence: B)
Class III
IV β blockers should not be administered to STEMI patients who have any of the following: (1) signs of heart failure, (2) evidence of low output state, (3) increased risk for cardiogenic shock, or (4) other relative contraindications to β blockade (PR interval greater than 0.24 seconds, second- or third-degree heart block, active asthma, or reactive airway disease). (Level of Evidence: B)
Morphine is frequently used for pain relief and is best administered intravenously in boluses of 1 to 2 mg, to a maximum of 10 to 15 mg for a normal adult. Respiratory depression can occur and care must be taken not to oversedate patients. Morphine should be used with caution in patients with hemodynamic instability because of its effects on reducing cardiac preload.
Several studies have demonstrated an increased risk of adverse cardiovascular events with the administration of nonsteroidal anti-inflammatory drugs (NSAIDS) and cyclooxygenase-2 inhibitors.34,35 These agents should not be administered for analgesia at any time during presentation with or hospitalization for STEMI. The Clinical Practice Guidelines recommend the following:24,33
Class I
Morphine sulfate (2-4 mg IV with increments of 2-8 mg IV repeated at 5- to 15-minute intervals) is the analgesic of choice for management of pain associated with STEMI. (Level of Evidence: C)
Patients routinely taking NSAIDS (except for aspirin), both nonselective as well as COX-2 selective agents, before STEMI should have those agents discontinued at the time of the presentation with STEMI because of the increased risk of mortality, reinfarction, hypertension, heart failure, and myocardial rupture associated with their use. (Level of Evidence: B)
Class III
NSAIDS (except for aspirin), both nonselective as well as COX-2 selective agents, should not be administered during hospitalization for STEMI because of the increased risk of mortality, reinfarction, hypertension, heart failure, and myocardial rupture associated with their use. (Level of Evidence: C)
Nitrates cause non–endothelium-dependent coronary vasodilatation, systemic venodilatation, reduced cardiac preload, and enhanced perfusion of ischemic myocardial zones (see Chap. 64). Animal and human data demonstrate that nitrate use may result in a reduction of infarct size; however, there is no benefit of nitrates in reducing mortality.36-38 For patients with symptomatic ischemia, however, intravenous nitroglycerin may be very effective, although nitroglycerin tolerance has been observed as early as 12 hours after institution of intravenous therapy. Often, hemodynamic intolerance to nitroglycerin may be a problem, especially in patients with inferior infarction, hemodynamically significant RV infarction, or concomitant aortic valve stenosis, or among the elderly, particularly in the setting of preexisting volume depletion. Often the hypotension is accompanied by a bradycardia as a manifestation of the von Bezold-Jarisch reflex.
Intravenous nitroglycerin should be initiated at 5 to 10 μg/min and gradually increased with a goal of a 10% to 30% reduction in systolic blood pressure and symptomatic pain relief. In most patients, use of this agent will be tapered within 24 to 36 hours. Nitrates should not be administered to patients with recent sildenafil use. In addition, approximately 40% of patients with an inferior STEMI will have involvement of the right ventricle, and nitrates should be used with caution in these patients in order to avoid profound hypotension, which usually responds promptly to intravenous volume replacement. The Clinical Practice Guidelines recommend the following:24
Class I
Patients with ongoing ischemic discomfort should receive sublingual nitroglycerin (0.4 mg) every 5 minutes for a total of 3 doses, after which an assessment should be made about the need for intravenous nitroglycerin. (Level of Evidence: C)
Intravenous nitroglycerin is indicated for relief of ongoing ischemic discomfort, control of hypertension, or management of pulmonary congestion. (Level of Evidence: C)
Class III
Nitrates should not be administered to patients with systolic blood pressure less than 90 mm Hg or greater than or equal to 30 mm Hg below baseline, severe bradycardia (<50 beats/min), tachycardia (>100 beats/min), or suspected RV infarction. (Level of Evidence: C)
Nitrates should not be administered to patients who have received a phosphodiesterase inhibitor for erectile dysfunction within the last 24 hours (48 hours for tadalafil). (Level of Evidence: B)
Anticoagulation with heparin is standard in the management of STEMI. Currently two forms of heparin are used: unfractionated heparin and low-molecular-weight heparin (LMWH). The use and pharmacology of these agents is explained in detail in Chap. 61.
When bound to antithrombin III, unfractionated heparin inactivates factor Xa and thrombin. Its use has been widely studied, and unfractionated heparin is considered a class I indication for patients with STEMI undergoing primary PCI or receiving fibrin-specific thrombolytic agents.24 An initial bolus of 60 U/kg (4000 U maximum) followed by a 12 U/kg/h (1000 U/h maximum) infusion should be administered promptly. A goal-activated partial thromboplastin time of 1.5 to 2.0 times normal should be achieved. The anticoagulation effects of unfractionated heparin, however, can be variable as a consequence of unpredictable protein binding.
The LMWHs are glycosaminoglycans consisting of chains of alternating residues of D-glucosamine and uronic acid. When compared with unfractionated heparin, they have a more predictable anticoagulation effect as a result of a longer half-life, better bioavailability, and dose-independent clearance. Unlike unfractionated heparin, the LMWHs have a great activity against factor Xa than thrombin, and their anticoagulation effect cannot be measured with standard laboratory tests.39
The use of the LMWH enoxaparin in conjunction with fibrinolysis has been studied in the ASSENT 3 (Assessment of the Safety and Efficacy of a New Thrombolytic Regimen) and TIMI-25 EXTRACT (Enoxaparin and Thrombolysis Reperfusion for Acute Myocardial Infarction Treatment) trials.40,41 In the ASSENT 3 trial, patients treated with enoxaparin plus tenecteplase had a lower combined end point of 30-day mortality, in-hospital reinfarction, and in-hospital refractory ischemia than did those treated with unfractionated heparin plus tenecteplase (11.4% vs 15.4%; P = .0002). The TIMI-25 EXTRACT trial randomized 20,506 patients receiving fibrinolytic therapy for STEMI to anticoagulation with enoxaparin through the entire index hospitalization or unfractionated heparin for the initial 48 hours. The combined primary end point of death or recurrent myocardial infarction at 30 days occurred in 9.9% of patients in the enoxaparin group and in 12% of those in the heparin group (P < .001).
Because of the delay in the onset of action of subcutaneous administration, an initial intravenous loading dose of 30 mg of enoxaparin followed by the traditional 1 mg/kg subcutaneous dose every 12 hours was used in both trials for patients younger than 75 years of age. Use of enoxaparin, however, was associated with a higher risk of minor and major bleeding when compared with unfractionated heparin (UFH) in the TIMI-25 EXTRACT trial.30
Direct thrombin inhibitors bind directly to thrombin and have been used as an alternative to heparin in patients with heparin-induced thrombocytopenia. Several studies have compared direct thrombin inhibitors with heparin for the management of STEMI patients.42,43 The Hirulog and Early Reperfusion or Occlusion (HERO)-2 trial randomized 17,073 STEMI patients to the direct thrombin inhibitor bivalirudin or heparin. Thirty-day mortality was similar in both groups; the incidence of reinfarction was decreased at the cost of an increased risk of mild and moderate bleeding.42 A meta-analysis of 11 randomized trials demonstrated that compared with heparin, direct thrombin inhibitors were associated with a lower risk of death or myocardial infarction both at the end of treatment (4.3% vs 5.1%; P = .001) and at 30 days (7.4% vs 8.2%; P = .02).44 This difference was primarily a result of a reduction in the incidence of reinfarction (2.8% vs 3.5%; P < .001) with no difference in mortality (1.9% vs 2.0%; P = .69). There was no excess in intracranial hemorrhage with direct thrombin inhibitors compared with heparins.44
The use of direct thrombin inhibitors in STEMI patients undergoing primary PCI was studied in The Harmonizing Outcomes with Revascularization and Stents in Acute Myocardial Infarction (HORIZONS-AMI).45 The trial randomized 3602 STEMI patients before undergoing primary PCI to bivalirudin alone or heparin plus a glycoprotein IIb/IIIa inhibitor. The combined primary end point of major bleeding as well as adverse clinical events (death, reinfarction, target-vessel revascularization, stroke) was lower in those treated with bivalirudin compared with those receiving heparin plus glycoprotein IIb/IIIa inhibitor (9.2% vs 12.1%; P = .005). The primary end point, however, was primarily driven by a reduction in major bleeding (4.9% vs 8.3%; P < .001). In addition, further subgroup analysis revealed that bivalirudin reduced the 30-day rate of cardiovascular death (1.8% vs 2.9%; P = .03) and all-cause death (2.1% vs 3.1%; P = .047). Use of bivalirudin, however, resulted in an increase in acute stent thrombosis within the first 24 hours compared with heparin plus glycoprotein IIb/IIIa (1.3 vs 0.3; P < .001). Thus bivalirudin is a reasonable alternative to treatment with heparin and GP IIb/IIIa inhibitors in a strategy of primary PCI for STEMI treatment.
The factor Xa inhibitor fondaparinux was investigated in the OASIS-6 (Organization for the Assessment of Strategies for Ischemic Syndromes) trial. In this study, 12,092 STEMI patients were randomized to fondaparinux for 8 days or placebo with standard heparin therapy.46 The primary end point of death or reinfarction at 30 days occurred in 9.7% of patients in the fondaparinux group and 11.2% of those in the placebo group (P = .008). Patients treated with fibrinolytic therapy had the most benefit, and there was no significant increase in the rate of bleeding.
In a subset of the OASIS-5 trial, fondaparinux was compared with enoxaparin in 20,078 patients with acute coronary syndrome, 6,238 of whom underwent PCI.47 Although similar rates of death, myocardial infarction, and stroke were seen between groups (6.2% vs 6.3% for the fondaparinux and enoxaparin, respectively; P = .79), the use of fondaparinux reduced major bleeding by one-half compared with enoxaparin (2.4% vs 5.1%; P < .00001). The rates of catheter-related thrombosis, however, were higher in those receiving fondaparinux alone compared with those receiving enoxaparin alone (0.9% vs 0.4%, respectively; hazard ratio = 2.3), which was completely obviated in both groups by the addition of UFH at the time of PCI without any increased risk of bleeding. Therefore, the use of upstream fondaparinux in patients with acute coronary syndrome undergoing PCI appears reasonable, although adjunctive UFH is recommended at the time of cardiac catheterization to prevent catheter-related thrombosis.
Class I
Patients undergoing reperfusion with fibrinolytics should receive anticoagulant therapy for a minimum of 48 hours (Level of Evidence: C) and preferably for the duration of the index hospitalization, up to 8 days (regimens other than UFH are recommended if anticoagulant therapy is given for more than 48 hours because of the risk of heparin-induced thrombocytopenia with prolonged UFH treatment). (Level of Evidence: A)
Anticoagulant regimens with established efficacy include:
UFH (initial intravenous bolus 60 U/kg [maximum 4000 U]) followed by an intravenous infusion of 12 U/kg/h (maximum 1000 U/h) initially, adjusted to maintain the activated partial thromboplastin time at 1.5 to 2.0 times control (approximately 50-70 seconds) (Level of Evidence: C). (Note: The available data do not suggest a benefit of prolonging the duration of the infusion of UFH beyond 48 hours in the absence of ongoing indications for anticoagulation; more prolonged infusions of UFH increase the risk of development of heparin-induced thrombocytopenia.)
Enoxaparin (provided the serum creatinine is <2.5 mg/dL in men and 2.0 mg/dL in women): for patients less than 75 years of age, an initial 30-mg intravenous bolus is given, followed 15 minutes later by subcutaneous injections of 1.0 mg/kg every 12 hours; for patients at least 75 years of age, the initial intravenous bolus is eliminated and the subcutaneous dose is reduced to 0.75 mg/kg every 12 hours. Regardless of age, if the creatinine clearance (using Cockroft-Gault formula) during the course of treatment is estimated to be less than 30 mL/min, the subcutaneous regimen is 1.0 mg/kg every 24 hours. Maintenance dosing with enoxaparin should be continued for the duration of the index hospitalization, up to 8 days. (Level of Evidence: A)
Fondaparinux (provided the serum creatinine is less than 3.0 mg/dL): initial dose 2.5 mg intravenously; subsequently subcutaneous injections of 2.5 mg once daily. Maintenance dosing with fondaparinux should be continued for the duration of the index hospitalization. (Level of Evidence: B)
For patients proceeding to primary PCI who have been treated with aspirin and a thienopyridine, recommended supportive anticoagulant regimens include the following:
For prior treatment with UFH, additional boluses of UFH should be administered as needed to maintain therapeutic activated clotting time levels taking into account whether glycoprotein IIa/IIIb receptor antagonists have been administered. (Level of Evidence: C) Bivalirudin may also be used in patients treated previously with UFH. (Level of Evidence: C)
For prior treatment with enoxaparin, if the last subcutaneous dose was administered within the prior 8 to 12 hours earlier, an intravenous dose of 0.3 mg/kg of enoxaparin should be given. (Level of Evidence: B)
For prior treatment with fondaparinux, administer additional intravenous treatment with an anticoagulant possessing anit-IIa activity, taking into account whether glycoprotein IIb/IIIa receptor antagonists have been administered. (Level of Evidence: C)
Bivalirudin is useful as a supportive measure for primary PCI with or without prior treatment with UFH. (Level of Evidence: B)
Class IIa
In patients with known heparin-induced thrombocytopenia, it is reasonable to consider bivalirudin as a useful alternative to heparin to be used in conjunction with streptokinase. Dosing according to the HERO-2 regimen (a bolus of 0.25 mg/kg followed by an intravenous infusion of 0.5 mg/kg/h for the first 12 hours and 0.25 mg/kg/h for the subsequent 36 hours)44 is recommended, but with a reduction in the infusion rate if the partial thromboplastin time is above 75 seconds within the first 12 hours. (Level of Evidence: B)
In STEMI patients undergoing PCI who are at high risk of bleeding, bivalirudin anticoagulation is reasonable. (Level of Evidence: B)
Class III
Because of the risk of catheter thrombosis, fondaparinux should not be used as the sole anticoagulant to support PCI. An additional anticoagulant with anti-IIa activity should be administered. (Level of Evidence: C)
The main goal of STEMI management is rapid reperfusion to establish coronary blood flow to ischemic myocardium. Currently, there are three main reperfusion strategies: fibrinolytic therapy, primary PCI, and fibrinolytic-facilitated primary PCI.
Fibrinolytic therapy for STEMI has been shown to be effective in numerous randomized trials involving more than 100,000 patients.48 It is widely available, easily administered, and is relatively inexpensive. However, only approximately 50% of STEMI patients are eligible for fibrinolytic therapy (Table 60–2), and only 50% to 60% of patients treated with fibrinolytics will achieve complete reperfusion (TIMI grade 3 flow). In addition, 10% to 20% of patients will experience reocclusion, and 1% will suffer from a stroke caused by intracranial hemorrhage. Fibrinolytic therapy is most effective when given within 3 hours from onset of chest pain.49
| Absolute contraindications |
| Any prior intracranial hemorrhage |
| Known structural cerebral vascular lesion |
| Known intracranial neoplasm |
| Ischemic stroke within the past 3 mo (except for acute stroke within 3 h) |
| Suspected aortic dissection |
| Active bleeding or bleeding diathesis (excluding menses) |
| Significant closed-head or facial trauma within 3 mo |
| Relative contraindications |
| History of chronic, severe, poorly controlled hypertension |
| Systolic pressure >180 mm Hg or diastolic 110 mm Hg |
| History of prior ischemic stroke >3 mo previously, dementia, or known intracranial pathology not covered in absolute contraindications |
| Recent (within 2-4 weeks) internal bleeding |
| Noncompressible vascular punctures |
| Pregnancy |
| Active peptic ulcer |
| Current use of anticoagulants: the higher the international normalized ratio, the higher the risk of bleeding |
| For streptokinase/anistreplase: prior exposure (>5 days previously) or prior allergic reaction to these agents |
Although streptokinase is still widely used around the world, fibrin-specific agents are almost exclusively used in the United States. These therapies are summarized in Table 60–3 and are molecular modifications of the tissue plasminogen activator (tPA) molecule. The impetus for their development was the hope that increasing fibrin specificity (TNK), or prolonging the plasma half-life (reteplase [rPA], tenecteplase [TNK], lanoteplase [nPA]) would increase TIMI grade 3 flow rates in the infarct-related artery beyond the approximately 50% found at 90-minute angiography for tPA. Phase 1 and 2 trials of these agents have shown TIMI grade 3 flow rates in excess of 60%; however, there was no significant reduction in mortality or in the incidence of stroke.50,51 The Clinical Practice Guidelines recommend the following:24
| Characteristic | Alteplase (tPA) | Reteplase (rPA) | Tenecteplase (tPA) | Lanoteplase (nPA) |
|---|---|---|---|---|
| Immunogenicity | No | No | No | ? |
| Plasminogen activation | Direct | Direct | Direct | Direct |
| Fibrin specificity | ++ | + | +++ | + |
| Plasma half-life | 4-6 min | 18 min | 20 min | 37 min |
| Dose | 15-mg bolus plus 90-min infusion up to 85 mg | 0+10-MU double bolus 30 min apart | ± 0.5 mg/kg single bolus | 120 KU/kg single bolus |
| PAI-1 resistance | No | ? | Yes | ? |
| Genetic alteration to native tPA | No | Yes | Yes | Yes |
| Recombinant version | Finger, EGF, and kringle-1 regions deleted | 2 single amino acid substitutions in kringle-1 and substitution of 4 amino acids in catalytic domain | Finger, EGF regions deleted and glycosylation sites in kringle-1 domain modified |
Class I
In the absence of contraindications, fibrinolytic therapy should be administered to STEMI patients with symptom onset within the prior 12 hours and ST-segment elevation greater than 0.1 mV in at least two contiguous precordial leads or at least two adjacent limb leads. (Level of Evidence: A)
In the absence of contraindications, fibrinolytic therapy should be administered to STEMI patients with symptom onset within the prior 12 hours and new or presumably new LBBB. (Level of Evidence: A)
Class IIa
In the absence of contraindications, it is reasonable to administer fibrinolytic therapy to STEMI patients with symptom onset within the prior 12 hours and 12-lead ECG findings consistent with a true posterior myocardial infarction. (Level of Evidence: C)
In the absence of contraindications, it is reasonable to administer fibrinolytic therapy to patients with symptoms of STEMI beginning within the prior 12 to 24 hours who have continuing ischemic symptoms and ST-segment elevation greater than 0.1 mV in at least two contiguous precordial leads or at least two adjacent limb leads. (Level of Evidence: B)
Class III
Fibrinolytic therapy should not be administered to asymptomatic patients whose initial symptoms of STEMI began more than 24 hours earlier. (Level of Evidence: C)
Fibrinolytic therapy should not be administered to patients whose 12-lead ECG shows only ST-segment depression except if a true posterior myocardial infarction is suspected. (Level of Evidence: A)
See Chap. 62 for a more detailed discussion. Approximately 95% of patients who are treated with primary PCI obtain complete reperfusion versus 50% to 60% of patients who are treated with fibrinolytics. Primary PCI is also associated with a lower risk of stroke than treatment with fibrinolysis, and diagnostic angiography quickly defines coronary anatomy, LV function, and mechanical complications.
A meta-analysis by Keeley et al52 of 23 trials that included 3872 patients treated with primary PCI and 3867 patients treated with fibrinolytics showed that PCI was superior to fibrinolytic therapy. Primary PCI was associated with a lower mortality rate (7% vs 9%; P = .0002), less reinfarction (3% vs 7%; P = .0001), and fewer strokes (1% vs 2%; P = .0004) at 30 days when compared with fibrinolysis. PCI capability, however, is only available at fewer than 50% of hospitals in the United States, and each 30-minute delay from symptom onset to balloon inflation during primary PCI is associated with a 7.5% relative increase in mortality at 1 year.53 In addition, a meta-analysis by Nallamothu and Bates54 of data from 23 trials that included 7739 patients, which compared fibrinolytic therapy with primary PCI, demonstrated that the mortality advantage of primary PCI is lost if the door-to-balloon time is 60 minutes greater than the door-to-needle time for fibrinolytic therapy.
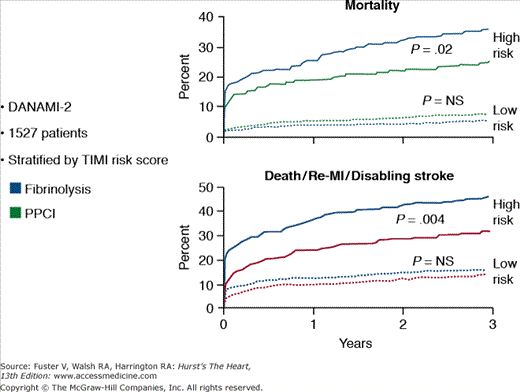
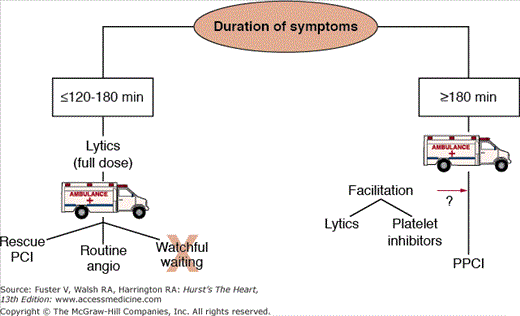
Three large-scale, randomized trials have compared fibrinolysis with transfer for primary PCI in STEMI patients (Table 60–4). The combined end point of death, reinfarction, and disabling stroke at 30 days was significantly lower in the patients treated with primary PCI. Although transfer for primary PCI appears to be the treatment of choice, two important points need to be made. First, the transport time in these trials was extremely short (median time in the DANAMI-2 [Danish Trial in Acute Myocardial Infarction-2] trial was 32 minutes), and these times may not be achievable outside of a clinical trial and in areas in which longer distances and weather may play a substantial role in hindering rapid transport. Second, fibrinolytic therapy still has a critically important role during the “golden hour” (Fig. 60–2) of myocardial infarction or when there is a delay in transfer.49 The mortality rates and infarct size in patients treated with thrombolytic therapy within the first 60 to 90 minutes of symptoms are extremely low, suggesting that fibrinolytic therapy still plays a vital role in the management of patients presenting to hospitals without primary PCI capability. Also, the benefit of primary PCI appears to be mainly in high-risk patients, which were a minority of the patients in the trial (Fig. 60–3).55 The Clinical Practice Guidelines recommend the following:24,33
| Death, Reinfarction, Disabling Stroke at 30 Days | ||||
|---|---|---|---|---|
| N | Thrombolysis (%) | Transfer for Pci (%) | P | |
| PRAGUE-2180 | 850 | 15.2 | 8.4 | <0.003 |
| AIR PAMI181 | 138 | 13.6 | 8.4 | 0.33 |
| DANAMI-2182 | 1572 | 13.7 | 8.0 | <0.001 |
Figure 60–3.
Risk stratification and the results of primary PCI in patients from the DANAMI 2 trial. MI, myocardial infarction; PPCI, primary percutaneous coronary intervention; TIMI, thrombolysis in myocardial infarction. Modified with permission from Thune et al.55
Class I
General considerations: If immediately available, primary PCI should be performed in patients with STEMI (including true posterior myocardial infarction) or myocardial infarction with new or presumably new LBBB who can undergo PCI of the infarct artery within 12 hours of symptom onset, if performed in a timely fashion (balloon inflation within 90 minutes of presentation) by persons skilled in the procedure (individuals who perform more than 75 PCI procedures per year). The procedure should be supported by experienced personnel in an appropriate laboratory environment (a laboratory that performs more than 200 PCI procedures per year, of which at least 36 are primary PCI for STEMI, and has cardiac surgery capability). (Level of Evidence: A)
Specific considerations:
STEMI patients presenting to a hospital with PCI capability should be treated with primary PCI within 90 minutes of first medical contact as a systems goal. (Level of Evidence: A)
STEMI patients presenting to a hospital without PCI capability and who cannot be transferred to a PCI center and undergo PCI within 90 minutes of first medical contact should be treated with fibrinolytic therapy within 30 minutes of hospital presentation as a systems goal unless fibrinolytic therapy is contraindicated. (Level of Evidence: B)
If the symptom duration is within 3 hours and the expected door-to-balloon time minus the expected door-to-needle time is:
- i. within 1 hour, primary PCI is generally preferred. (Level of Evidence: B)
- ii. greater than 1 hour, fibrinolytic therapy (fibrin-specific agents) is generally preferred. (Level of Evidence: B)
- i. within 1 hour, primary PCI is generally preferred. (Level of Evidence: B)
If symptom duration is greater than 3 hours, primary PCI is generally preferred and should be performed with a medical contact-to-balloon or door-to-balloon interval as short as possible and a goal of within 90 minutes. (Level of Evidence: B)
Primary PCI should be performed for patients younger than 75 years old with ST-segment elevation or LBBB who develop shock within 36 hours of myocardial infarction and are suitable for revascularization that can be performed within 18 hours of shock unless further support is futile because of the patient’s wishes or contraindications/unsuitability for further invasive care. (Level of Evidence: A)
Primary PCI should be performed in patients with severe congestive heart failure and/or pulmonary edema (Killip class 3) and onset of symptoms within 12 hours. The medical contact-to-balloon or door-to-balloon time should be as short as possible (ie, goal within 90 minutes). (Level of Evidence: B)
Class IIa
Primary PCI is reasonable for selected patients age 75 years or older with ST-segment elevation or LBBB or who develop shock within 36 hours of myocardial infarction and are suitable for revascularization that can be performed within 18 hours of shock. Patients with good prior functional status who are suitable for revascularization and agree to invasive care may be selected for such an invasive strategy. (Level of Evidence: B)
It is reasonable to perform primary PCI for patients with onset of symptoms within the prior 12 to 24 hours and one or more of the following:
Severe congestive heart failure (Level of Evidence: C)
Hemodynamic or electrical instability (Level of Evidence: C)
c. Persistent ischemic symptoms. (Level of Evidence: C)
Class IIb
The benefit of primary PCI for STEMI patients eligible for fibrinolysis is not well established when performed by an operator who performs fewer than 75 PCI procedures per year. (Level of Evidence: C)
Class III
PCI should not be performed in a noninfarct artery at the time of primary PCI in patients without hemodynamic compromise. (Level of Evidence: C)
Primary PCI should not be performed in asymptomatic patients more than 12 hours after onset of STEMI if they are hemodynamically and electrically stable. (Level of Evidence: C)
Facilitated PCI refers to the pretreatment with fibrinolytics in STEMI patients as a bridge to intended immediate PCI. This pretreatment was proposed as a method to initiate earlier reperfusion and reduce ischemic time and infarct size in patients who experience a delay before the onset of PCI. Fibrinolytic-facilitated therapy, however, can expose patients to a higher risk of bleeding, including intracranial bleeding. Two large trials of facilitated PCI, ASSENT-4 and FINESSE (Facilitated Intervention with Enhanced Reperfusion Speed to Stop Events), have investigated the use of fibrinolytic-facilitated PCI in STEMI. The ASSENT 4 trial randomized 1666 STEMI patients undergoing immediate PCI to pretreatment with a fibrinolytic (tenecteplase) or placebo. The data safety monitoring board halted the trial before the enrollment of the 4000 anticipated patients because of an increased 30-day mortality rate in the facilitated PCI group (6.0% vs 3.8%; P = .004).56 Patients in the fibrinolytic plus PCI group also had a greater incidence of stroke (1.81% vs 0%; P < .001), bleeding (31.3% vs 23.4%; P < .001), and reinfarction (4.1% vs 1.9%; P = .01).
In the FINESSE trial, 2452 STEMI patients undergoing immediate PCI were randomized to receive one of the following three strategies: half-dose fibrinolytic (reteplase) plus the glycoprotein IIb/IIIa inhibitor abciximab (combination-facilitated PCI), early administration of abciximab (abciximab-facilitated PCI), or late administration of abciximab (primary PCI).57 A primary composite end point that included death from all causes and complication of myocardial infarction through day 90 showed no significant differences among all three groups (9.8%, 10.5%, and 10.7%, respectively; P = .55). Safety end points indicated a significant difference between the combination-facilitated PCI and primary-PCI groups, showing increased nonintracranial TIMI major or minor bleeding (14.5% vs 6.9%; P < .001). Treatment with early abciximab was associated with a trend toward increased bleeding.
Therefore, based on the results of ASSENT-4 and FINESSE, the data do not support the use of facilitated PCI as a routine reperfusion strategy in STEMI.
Unlike facilitated PCI, in which PCI is performed immediately after fibrinolysis, pharmacoinvasive strategies refer to PCI that is routinely performed between 2 and 24 hours after fibrinolysis. The TRANSFER-AMI (Trial of Routine Angioplasty and Stenting after Fibrinolysis to Enhance Reperfusion in Acute Myocardial Infarction) is the largest and most recent among five studies that have evaluated routine early PCI after fibrinolysis.58 In this study, 1059 STEMI patients who received fibrinolytic therapy were randomized to either standard treatment (transfer for angiography no less than 24 hours after lysis) or PCI of the infarct-related artery within 6 hours of fibrinolysis (“early PCI”). The primary end point was a composite of death, reinfarction, recurrent ischemia, new or worsening heart failure, or cardiogenic shock. At 30 days, the primary end point occurred in 11% of the early PCI group versus 17.2% of the standard group (P = .004). The favorable results for the early PCI group were primarily driven by a decreased rate of reinfarction. Notably, groups did not differ in the incidence of major bleeding.
The results of the four smaller studies investigating early PCI after fibrinolysis (CAPITAL AMI [Combined Angioplasty and Pharmacological Intervention Versus Thrombolytics Alone in Acute Myocardial Infarction],59 CARESS-in-AMI [Combined Abciximab REteplase Stent Study in Acute Myocardial Infarction],60 SIAM III [Southwest German Interventional Study in Acute Myocardial Infarction],61 and GRACIA [Grupo de Análisis de la Cardiopatía Isquémica Aguda] 162) are consistent with the findings of TRANSFER-AMI. However, the optimal window to perform PCI after receiving fibrinolytic therapy is still uncertain. The interval between fibrinolysis and PCI in the aforementioned studies ranged from 2 to 17 hours, with each interval suggesting similar efficacy.63 After fibrinolysis, the risk of reocclusion increases within 24 hours. Therefore, until further data are available, striving for early PCI between 2 and 24 hours appears to be most reasonable.
For STEMI patients, when available, primary PCI is the preferred reperfusion strategy. However, when this therapy is not available, fibrinolysis followed by PCI within 2 to 24 hours appears to be a reasonable alternative.
Class IIa
It is reasonable for high-risk patients who receive fibrinolytic therapy as primary reperfusion therapy at a non–PCI-capable facility to be transferred as soon as possible to a PCI-capable facility where PCI can be performed either when needed or as a pharmacoinvasive strategy. Consideration should be given to initiating a preparatory antithrombotic (anticoagulant plus antiplatelet) regimen before and during patient transfer to the catheterization laboratory. (Level of Evidence: B)
Class IIb
Patients who are not at high risk who receive fibrinolytic therapy as primary reperfusion therapy at a non–PCI-capable facility may be considered for transfer as soon as possible to a PCI-capable facility where PCI can be performed either when needed or as a pharmacoinvasive strategy. Consideration should be given to initiating a preparatory antithrombotic (anticoagulant plus antiplatelet) regimen before and during patient transfer to the catheterization laboratory. (Level of Evidence: C)
Class III
A planned reperfusion strategy using full-dose fibrinolytic therapy followed by immediate PCI may be harmful. (Level of Evidence: B)
Approximately 40% to 50% of patients who receive fibrinolytic therapy fail to achieve optimal reperfusion, and 20% suffer reinfarction within the first 12 hours. The optimal management of these patients was investigated in the REACT (Rescue Angioplasty versus Conservative Treatment or Repeat Thrombolysis) trial, which randomized 427 patients who failed to achieve reperfusion with fibrinolysis to repeat fibrinolysis, conservative therapy, or rescue PCI.64
The combined primary end point of death, reinfarction, stroke, or severe heart failure within 6 months was lower in the rescue PCI group (15.3%) compared with those in the repeat fibrinolysis and conservative management groups (31% and 29.8%, respectively; P < .001). The primary end point was mainly driven by a decrease in recurrent myocardial infarction in the rescue PCI group compared with the repeat fibrinolysis and conservative management groups (2.1% vs 10.6% and 8.5%, respectively; P < .01). There was no difference in major bleeding among groups, though a significant increase in minor bleeding with rescue PCI was observed (P < .001), due mainly to sheath-related bleeding.
Recently, longer-term outcomes of the REACT trial were reported, with the results indicating that rescue PCI remains superior to more conservative, noninvasive strategies.65 One-year clinical follow-up was available in 91% of the 427 randomized patients. At 1 year, event-free survival for patients was 81.5% for rescue PCI compared with 64.1% and 67.5% for repeat fibrinolysis and conservative therapy, respectively (P = .004). More importantly, a significant reduction in long-term mortality was seen in the rescue PCI group. At 4.4 years from randomization, there were 77 total deaths; 11.2% were from the rescue PCI group compared with 22.3% and 22.4% from the repeat fibrinolysis and conservative therapy groups (P = .026).
These and other data support the recommendation that rescue PCI should be considered for patients in whom fibrinolytic therapy fails to achieve reperfusion in STEMI. The Clinical Practice Guidelines state the following:24
Class I
Rescue PCI should be performed in patients less than 75 years old with ST-segment elevation or LBBB who develop shock within 36 hours of myocardial infarction and are suitable for revascularization that can be performed within 18 hours of shock unless further support is futile because of the patient’s wishes or contraindications/unsuitability for further invasive care. (Level of Evidence: B)
Rescue PCI should be performed in patients with severe CHF and/or pulmonary edema (Killip class 3) and onset of symptoms within 12 hours.(Level of Evidence: B)
Class IIa
Rescue PCI is reasonable for selected patients 75 years or older with ST-segment elevation or LBBB or who develop shock within 36 hours of myocardial infarction and who are suitable for revascularization that can be performed within 18 hours of shock. Patients with good prior functional status who are suitable for revascularization and who agree to invasive care may be selected for such an invasive strategy. (Level of Evidence: B)
It is reasonable to perform rescue PCI for patients with 1 or more of the following:
Hemodynamic or electrical instability (Level of Evidence: C)
Persistent ischemic symptoms (Level of Evidence: C)
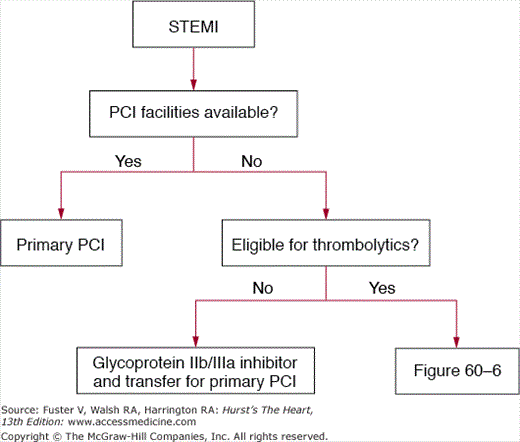
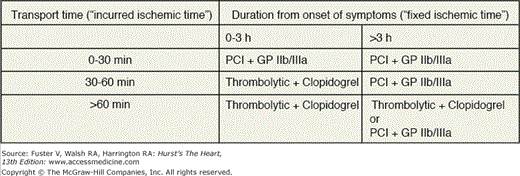
Figures 60–4, 60–5, and 60–6 outline a systematic, evidence-based framework for selecting reperfusion strategies in STEMI patients.66 STEMI patients presenting to PCI-capable hospitals should undergo primary PCI with a target door-to-balloon time of less than 90 minutes. If the hospital does not have PCI capability, the clinician must first determine whether the patient is eligible for fibrinolytic therapy (see Table 60–2). Patients ineligible for fibrinolytic therapy should be transferred for primary PCI. For those who are eligible for fibrinolytic therapy, the clinician must then consider two important factors: duration from onset of symptoms (fixed ischemia time) and transport time to the nearest PCI facility (incurred ischemia time). These two factors can be incorporated into a 2 × 3 table to select a reperfusion strategy (see Fig. 60–6).
Figure 60–6.
Table organizing the treatment strategies for thrombolytic eligible ST-segment elevation myocardial infarction patients who present to hospitals without facilities for percutaneous coronary intervention. GPIIb/IIIa, glycoprotein IIb/IIIa inhibitor; lytics, thrombolysis (patients should be immediately transferred to a PCI facility after thrombolysis); PCI, transfer for percutaneous coronary intervention.
Patients facing a transport time of less than 30 minutes should be transferred for primary PCI. Fibrinolytic-eligible patients who present less than 2 to 3 hours from onset of symptoms and have a transport time precluding primary PCI within 90 minutes of first medical contact should receive fibrinolytic therapy. Patients presenting more than 2 to 3 hours after the onset of chest pain and have a transport time of 60 minutes or less should be promptly transported for primary PCI. If the anticipated transport time is more than 60 minutes, patients can be treated with either fibrinolytic therapy or primary PCI.
Duration of symptoms and time to treatment play a crucial role in the decision to administer fibrinolytics or to proceed with transfer for primary PCI. For those eligible to receive fibrinolytics, prehospital administration (as opposed to in-hospital administration) of fibrinolytic therapy is a means to decrease time to reperfusion. The CAPTIM (Comparison of Angioplasty and Prehospital Thrombolysis in Acute Myocardial Infarction) trial is the only study to compare prehospital fibrinolysis and primary PCI as a function of time from onset of symptoms. The trial randomized 840 STEMI patients to prehospital fibrinolysis or primary PCI. Outcomes were assessed according to time between symptom onset (<2 hours or >2 hours) and randomization to each group. Results indicated that regardless of time of symptom onset, there was no significant difference between prehospital fibrinolysis and primary PCI in the primary end point of combined death, reinfarction, or disabling stroke (7.4% vs 6.5% [P = .855] in those with <2 hours symptom onset and 9.1% vs 5.9% [P = .326] in those with >2 hours symptom onset). There was, however, a trend toward increased mortality in those randomized within 2 hours who received primary PCI versus fibrinolytic therapy (5.7% vs 2.2%; P = .058). This trend was almost completely reversed in those randomized after 2 hours (7% for PCI vs 11% for fibrinolysis; P = .470). Although patients randomized early who underwent primary PCI also experienced significantly more cardiogenic shock (5.3% vs 1.3%; P = .032), this secondary end point was driven primarily by transport time.
The results of CAPTIM are consistent with those of PRAGUE-2, which showed similar mortality rates for fibrinolysis and primary PCI when patients presented within 3 hours of symptom onset, but a higher mortality with fibrinolysis when patients were randomized after 3 hours. Long-term mortality data of CAPTIM were recently published, indicating outcomes consistent with those of the initial study.67
These data suggest that duration of symptoms should be a strong consideration in the selection of prehospital fibrinolytic versus primary PCI therapy for STEMI. Barring exclusion criteria, in patients experiencing a symptom duration of less than 2 to 3 hours, prehospital fibrinolysis may be a viable option if there is an anticipated transfer time of greater than 60 minutes.
All patients receiving fibrinolytic therapy should be transferred to a PCI facility for potential failure to achieve reperfusion (ongoing chest pain or <50% resolution of ST-segment elevation at 90 minutes) and rescue PCI. Recent randomized data suggest that all STEMI patients who are treated with fibrinolytic therapy benefit from routine coronary angiography during the index hospitalization.68
The Clinical Practice Guidelines state the following:33
Class IIb
Coronary angiography may be considered as part of an invasive strategy for risk assessment after fibrinolytic therapy (Level of Evidence: B) or for patients not undergoing primary reperfusion. (Level of Evidence: C)
Clopidogrel is an oral thienopyridine prodrug whose active metabolite inhibits the activation of platelets by adenosine diphosphate. Its antiplatelet effects are more potent than aspirin and less potent than the glycoprotein IIb/IIIa inhibitors.
Clopidogrel in combination with fibrinolytic therapy has been studied in the CLARITY-TIMI 28 (Clopidogrel as Adjunctive Reperfusion Therapy–Thrombolysis in Myocardial Infarction 28) trial.69 In this trial, 3491 STEMI patients 75 years of age or younger who were treated with thrombolytics were randomized to therapy with aspirin plus placebo or aspirin plus clopidogrel. Clopidogrel was given as a 300-mg loading dose within minutes of thrombolysis and 75 mg daily thereafter. The composite primary end point of death, reinfarction before angiography, or occluded infarct-related artery at angiography occurred in 15% of patients in the clopidogrel group and in 22% of patients in the placebo group (P < .001). The use of clopidogrel was not associated with a higher rate of major or minor bleeding.
Although a loading dose of 300 mg of clopidogrel was not associated with a greater risk of bleeding, patients older than 75 years of age were excluded from the study. Patients older than 75 years of age were included in the COMMIT32 (a randomized, placebo-controlled trial of adding clopidogrel to aspirin in 46,000 AMI patients), which randomized 45,849 STEMI patients treated with fibrinolysis to therapy with aspirin plus placebo or aspirin plus clopidogrel. Unlike the CLARITY trial, a 300-mg loading dose was not given. Instead, patients randomized to the clopidogrel arm received 75 mg of clopidogrel at the time of fibrinolysis and then 75 mg daily for the duration of hospitalization. Patients in the clopidogrel arm had a lower rate of the composite end point of death, reinfarction, or stroke (9.3% vs 10.1%; P = .002) and no increase in major or minor bleeding.
The data from these two trials suggest that patients treated with fibrinolysis should receive clopidogrel. For patients older than 75 years of age, 75 mg of clopidogrel without a loading dose should be used. In patients 75 years of age or younger, the current data suggest that a 300-mg loading dose followed by 75 mg daily of clopidogrel is beneficial and safe.
Data from the PCI-CURE (Clopidogrel in Unstable Angina to Prevent Recurrent Ischemic Events) trial (effects of pretreatment with clopidogrel and aspirin followed by long-term therapy in patients undergoing PCI)70 demonstrated that the early use of clopidogrel in non–ST-segment elevation acute coronary syndrome patients who eventually undergo PCI is of benefit (see Chap. 62). The effectiveness of clopidogrel in the setting of primary PCI for STEMI is unproven, but extrapolation of the data indicating that upstream clopidogrel dosing improves PCI outcomes in non–ST-segment elevation myocardial infarction and scheduled PCI has led to the recent reasonable recommendation for oral clopidogrel loading at STEMI presentation and long-term maintenance therapy, barring contraindications in those younger than age 75.33
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree