Sedatives, Analgesics, and Paralytics
Learning Objectives
On completion of this chapter, the reader will be able to do the following:
1 List the most common sedatives and analgesics used in the treatment of critically ill patients.
3 Describe the most common method for assessing the need for and level of sedation.
6 Discuss the mode of action of depolarizing and nondepolarizing paralytics.
9 Recommend a medication for a mechanically ventilated patient with severe anxiety and agitation.
Key Terms
• Analgesic
• Anesthetic
• Anterograde amnesic
• Depolarizing agents
• Miosis
• Nondepolarizing agents
• Paralytic
• Pruritus
• Ramsay Sedation Scale
• Sedative
• Train-of-four monitoring
Sedatives, analgesics, and paralytics are often required for the treatment of mechanically ventilated patients in the intensive care unit (ICU). The importance of these drugs in the management of critically ill patients requires critical care therapists to have a working knowledge of the indications and contraindications, mode of action, potential adverse effects, and the most appropriate methods to monitor the effects of these drugs.
Sedatives are used to reduce anxiety and agitation and to promote sleep and anterograde amnesia; analgesics are used to lessen pain. Paralytics are used to facilitate invasive procedures (e.g., surgery, endotracheal intubation) and to prevent movement and ensure the stability of artificial airways. Paralysis may also be used to decrease mean airway pressure ( ) during uncoordinated or uncontrolled mechanical ventilation.1–3
) during uncoordinated or uncontrolled mechanical ventilation.1–3
A variety of pharmacologic agents are available for achieving sedation and paralysis of mechanically ventilated patients. The most common sedative drugs used in the ICU include the following: (1) benzodiazepines (e.g., diazepam, midazolam, and lorazepam), (2) neuroleptics (e.g., haloperidol), (3) anesthetic agents (e.g., propofol), and (4) opioids (e.g., morphine, fentanyl). Paralysis can be achieved with neuromuscular blocking agents (NMBA) that are classified as depolarizing and nondepolarizing, depending on their mode of action. Succinylcholine is the only example of a depolarizing NMBA in widespread use; the most commonly used nondepolarizing NMBAs include pancuronium, vecuronium, and atracurium.
Maintaining an optimal level of comfort and safety for the patient should be a primary goal when administering sedatives, analgesics, and NMBAs. It is important, therefore, to recognize that although these agents can dramatically improve patient outcomes in mechanically ventilated patients, they can also precipitate significant hemodynamic, autonomic, and respiratory consequences in these patients (Key Point 15-1).
Sedatives and Analgesics
Sedation practices vary considerably because of institutional bias and because the requirements for sedation can vary greatly among patients.4 As mentioned, sedation is generally prescribed for critically ill patients to treat anxiety and agitation and to prevent or at least minimize sleep deprivation. Agitation and sleep deprivation can result from a variety of factors, including extreme anxiety, delirium, pain, and adverse drug effects. Sedation is also often required for mechanically ventilated patients who are being treated with nonconventional modes of ventilation, such as high-frequency ventilation, inverse inspiratory-to-expiratory ratio ventilation, and permissive hypercapnia.5
The Joint Commission has defined four levels of sedation: minimal, moderate, deep, and anesthesia (Box 15-1). It is important to recognize that sedation needs may vary considerably during the course of a patient’s stay in the ICU. For example, deeper levels of sedation and analgesia may be required during the initial phases of mechanical ventilation, especially in cases in which the patient is asynchronous or “fighting” the mechanical ventilatory mode being used. Conversely, minimal levels of sedation and analgesia are usually required during the recovery phase of an illness. Indeed, weaning a patient from mechanical ventilation can be severely hindered if the patient is oversedated.6 It should be apparent, therefore, that reliable and accurate methods for assessing the need and level of sedation and analgesia are essential for the successful management of critically ill patients.7
Monitoring the Need for Sedation and Analgesia
Several techniques have been proposed to assess the level of sedation in adults and children. Examples of scoring systems that have been validated for use in critically ill patients include the Ramsay Sedation Scale (RSS), the Motor Activity Assessment Scale (MAAS), the Sedation-Agitation Scale (SAS), and the Comfort Scale. Although considerable debate exists over the best technique, it is generally agreed that patients should be assessed regularly to ensure that they are relaxed and are not complaining of pain (Key Point 15-2).
The RSS is shown in Table 15-1.8 Notice that it is a graduated single-category scale. The grade assigned by the observer depends on the patient’s response to stimuli. The advantages of using this type of single category scale are that it is relatively easy to perform and provides a numerical value that can be used as a target for achieving adequate sedation. For example, a score of 2 to 4 on the RSS indicates adequate sedation. There are several disadvantages associated with using this type of graded scale. Most notably, it does not provide any guidance on selection of the most appropriate sedative, and it is a subjective, nonlinear scale that does not allow for consideration of changing physiological and psychological needs of a patient during the course of his or her illness.1
TABLE 15-1
| Score | Description |
| 1 | Patient is awake but anxious, agitated, and restless. |
| 2 | Patient is awake, cooperative, oriented, and tranquil. |
| 3 | Patient is semi-asleep but responds to verbal commands. |
| 4 | Patient is asleep and has a brisk response to a light glabellar tap or loud auditory stimulus. |
| 5 | Patient is asleep and has a sluggish response to a light glabellar tap or loud auditory stimulus. |
| 6 | Patient is asleep and has no response to a light glabellar tap or loud auditory stimulus. |
Benzodiazepines
Benzodiazepines have been the drugs of choice for the treatment of anxiety in critical care.1 Preferential use of these drugs by critical care physicians is probably related to their relatively low cost and to the ability of these drugs to produce anxiolytic, hypnotic, muscle relaxation, anticonvulsant, and anterograde amnesic effects. Anterograde amnesia relates to preventing the acquisition and encoding of new information that can potentially lead to memories of unpleasant experiences and posttraumatic stress disorder (PTSD).
Benzodiazepines exert their effects through a nonspecific depression of the central nervous system (CNS). This is accomplished when these drugs bind to benzodiazepine sites on the γ-aminobutyric acid (GABA) receptor complex on neurons in the brain. Binding of benzodiazepines to the GABA receptor complex increases the chloride permeability of the neuron, which in turn hyperpolarizes the neuron, making depolarization less likely.9
Benzodiazepines vary in potency, onset of action, uptake, distribution, and elimination half-life (see Table 15-2 for a comparison of the pharmacologic properties of diazepam, midazolam, and lorazepam.) It is worth noting that the intensity and duration of action for the various benzodiazepines can be affected by a number of patient-specific factors, including age, underlying pathology, and concurrent drug therapy. Prolonged recovery from benzodiazepines typically occurs in patients with renal and hepatic insufficiency.7
TABLE 15-2
Selected Sedatives Used for Critically Ill Adult Patients
| Agent | Onset After IV Dose (Min) | Half-Life of Parent Compound (h) | Intermittent IV Dose | Infusion Dose Range (Usual) |
| Diazepam | 2-5 | 20-120 | 0.03-0.1 mg/kg q 0.5-6 h | — |
| Midazolam | 2-5 | 3-11 | 0.02-0.08 mg/kg q 0.5-2 h | 0.04-0.2 mg/kg/h |
| Lorazepam | 5-20 | 8-15 | 0.02-0.06 mg/kg q 2-6 h | 0.01-0.1 mg/kg/h |
| Propofol | 1-2 | 26-32 | — | 5-80 µg/kg/min |
| Haloperidol | 3-20 | 18-54 | 0.03-0.15 mg/kg q 0.5-6 h | 0.04-0.15 mg/kg/h |
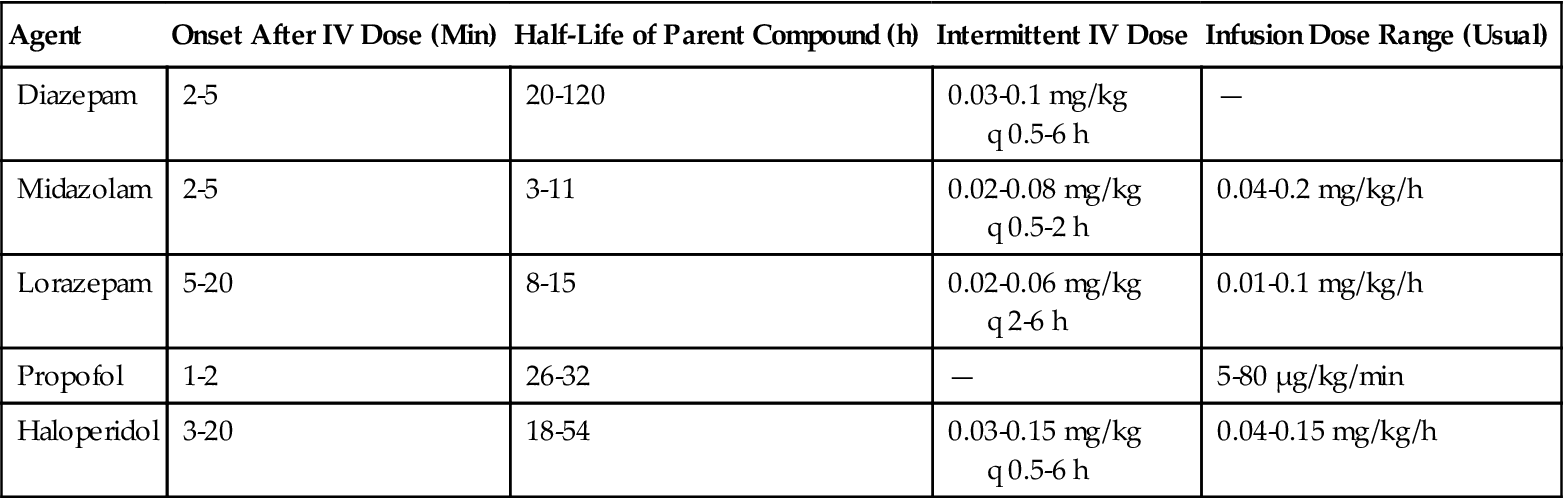
Modified from Jacobi J, Fraser GL, Coursin DB, et al: Clinical practice guidelines for the sustained use of sedatives and analgesics in the critically ill adult, Crit Care Med 30(1) 119-141, 2002.
Benzodiazepines generally produce only minimal effects on cardiovascular function; however, they can cause a significant drop in blood pressure when initially administered to hemodynamically unstable patients (e.g., patients with hypovolemic shock). Similarly, benzodiazepines normally do not adversely affect the respiratory system; however, they can produce hypoventilation or apnea by causing a reduction in ventilatory drive in patients with chronic obstructive pulmonary disease (COPD) when combined with opioids.
Reversal of the effects of benzodiazepines can be accomplished with flumazenil (Romazicon), which prevents the sedative effects of these drugs by competitively binding to benzodiazepine receptors. It is a short-acting drug that is administered intravenously at doses of 0.2 to 1.0 mg; subsequent doses may be repeated every 20 minutes up to a maximum dose of 3 mg/h. Administration of flumazenil is generally reserved for patients admitted to the emergency department for suspected benzodiazepine overdose. The most common side effects of flumazenil include dizziness, panic attacks, and cardiac ischemia, and it may lead to seizures in patients receiving long-term benzodiazepine or tricyclic antidepressant therapy.
Diazepam
Diazepam (Valium) has a rapid onset of action because of its high lipid solubility and ability to traverse the blood-brain barrier relatively quickly. The average onset of action for diazepam when it is administered intravenously is 3 to 5 minutes.9 It is metabolized in the liver to active metabolites that have relatively long half-lives (40-100 hours). These active metabolites are ultimately eliminated by the kidney. As such, diazepam elimination can be decreased in older patients, neonates, and patients with compromised hepatic and renal function, resulting in prolonged clinical effects and delayed recovery from sedation.10
Intravenous (IV) administration of diazepam is the most reliable method to maintain sedation in critically ill patients because absorption through the oral and intramuscular routes can vary considerably. Continuous infusion of diazepam is not recommended. Instead, a bolus dose of the drug is administered at the start of an infusion, followed by a series of smaller boluses with close titration to produce the desired plasma concentration of the drug.11
Midazolam
Midazolam (Versed) has a rapid onset of action and short half-life, making it an ideal sedative for the treatment of acutely agitated patients (Key Point 15-3). Note that although it does have a short half-life, prolonged sedation can occur as a result of the accumulation of the drug and its metabolites in the peripheral tissues when it is administered for longer than 48 hours.1
Midazolam causes a reduction in cerebral perfusion pressure, but it does not protect against increases in intracranial pressure for patients receiving ketamine.1 Although midazolam does not cause respiratory depression in most patients, it depresses sensitivity of upper respiratory reflexes, and it can reduce the ventilatory response in patients with COPD and in patients receiving narcotics.12
Midazolam typically causes only minimal hemodynamic effects (e.g., lower blood pressure, reduction in heart rate) in euvolemic subjects and is usually well tolerated in patients with left ventricular dysfunction. It can produce significant reductions in systemic vascular resistance and blood pressure in patients who are dependent on increased sympathetic tone to maintain venous return.1
Lorazepam
Lorazepam (Ativan) is the drug of choice for sedating mechanically ventilated patients in the ICU for longer than 24 hours. It has a slower onset of action compared with diazepam and midazolam due to its lower lipid solubility and longer time required to cross the blood-brain barrier. Its lower lipid solubility coupled with decreased distribution in peripheral tissues may account for its longer duration of action in some patients when compared with diazepam and midazolam.13
Potential adverse drug interactions are less likely with lorazepam than with other benzodiazepines because it is metabolized in the liver to inactive metabolites. Continual use of lorazepam, however, has been associated with several side effects, including lactic acidosis, hyperosmolar coma, and a reversible nephrotoxicity. These latter side effects have been attributed to the use of the solvents propylene glycol and polyethylene glycol in the manufacture of lorazepam.9 It is also worth noting that lorazepam acts synergistically with other central CNS depressants and should be administered with caution in patients receiving these drugs.13 Case Study 15-1 provides more information about several potential harmful effects associated with long-term use of lorazepam.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


