Data from randomized clinical trials have shown the safety and efficacy of the XIENCE V in selected populations. However, limited data are available comparing the XIENCE V to the first-generation CYPHER sirolimus-eluting stent. This study aimed to assess the long-term safety and clinical efficacy of the XIENCE V everolimus-eluting stent compared to first-generation stents in an unselected patient population. This retrospective analysis included 6,069 patients treated with CYPHER, TAXUS, and XIENCE stents from 2003 to 2009 at our institution. The patients were followed up for ≥1 year after the index procedure. The baseline characteristics were generally comparable among the 3 groups, with the exception of a significantly greater prevalence of diabetes mellitus, systemic hypertension, and a history of angioplasty and coronary bypass surgery among the XIENCE patients. The XIENCE patients also had a twofold greater rate of type C lesions. One-year follow-up data were available for 82% of the patients. The 1-year major adverse cardiovascular events rate was 9.3% for the XIENCE stent versus 9.8% for the CYPHER stent and 11.5% for the TAXUS stent (p = 0.11). Mortality was lower in the XIENCE group than in the CYPHER and TAXUS groups (3.6% vs 4.9% vs 7.2%, respectively, p <0.001), and target lesion revascularization was similar (5.9% vs 5.2% vs 5.6%, respectively; p = 0.34). Stent thrombosis was lower in the XIENCE patients (0.2% vs 1.2% vs 0.7%, p = 0.007). In conclusion, in a contemporary United States clinical practice with an unselected patient population, use of the XIENCE V stent was associated with an improved safety profile and reduction of all-cause mortality and stent thrombosis compared to first-generation drug-eluting stents. The XIENCE V failed to demonstrate superiority for overall major adverse cardiovascular events, Q-wave myocardial infarction, and revascularization rates.
The introduction of first-generation drug-eluting stents (DES), namely the sirolimus-eluting CYPHER stent (Cordis, Miami Lakes, Florida) and the paclitaxel-eluting TAXUS stent (Boston Scientific, Natick, Massachusetts), has led to dramatic reductions in in-stent restenosis rates. Restenosis, however, has remained a concern with these first-generation stents, and events of late and very late stent thrombosis have emerged as significant hazards, necessitating prolonged dual antiplatelet therapy. These limitations led to the development of second-generation DESs with superior designs incorporating biocompatible polymers, thinner struts, and new stent alloys.
The XIENCE V everolimus-eluting stent (EES) (Abbott Vascular, Santa Clara, California) is a second-generation DES designed on the Multilink Vision L-605 cobalt-chromium alloy balloon expandable stent with a 0.0032-in. strut thickness enveloped with a conformal coating of anon-erodible biocompatible polymer loaded with 100 μg/cm 2 everolimus. It is designed to release 80% of the drug within 30 days, with nearly all the drug released within 4 months.
Data from the Clinical Evaluation of the XIENCE V Everolimus-Eluting Coronary Stent System in the Treatment of Patients with de novo Coronary Artery Lesions (SPIRIT) randomized trial series showed the safety and efficacy of the XIENCE V in selected populations and led to its approval in the United States in 2008. Additional safety and long-term efficacy data have accumulated from international registries and trials. The present study assessed the long-term safety and clinical effectiveness of the XIENCE V EES compared to the first-generation stents in an unselected patient population.
Methods
The treatment group included all patients who received ≥1 XIENCE V EESs at our institution from 2003 to 2009 as a part of the Registry Experience at the Washington Hospital Center, DES–XIENCE V (REWARDS XV) trial. The 2 control groups included patients who received a sirolimus-eluting CYPHER stent or a paclitaxel-eluting TAXUS stent during the study period. Patients who received an EES other than XIENCE V or a bare metal stent at the initial XIENCE V stent implantation were excluded. All patients provided written informed consent. The study complied with the Declaration of Helsinki for investigation in humans and was approved by the institutional ethics committee.
The interventional strategy and the use of adjunctive devices and pharmacotherapy were at the discretion of the operating interventional cardiologist. All patients received aspirin 325 mg before the procedure and were recommended to continue this regimen indefinitely. In addition, clopidogrel 75 mg/day after a 300- or 600-mg loading dose was begun before the procedure and subsequently continued for 12 months. The cardiac history and baseline demographics were prospectively collected, along with the angiographic data; however, data analysis was performed retrospectively. Patients were followed up by telephone interview or survey for all major adverse cardiovascular events (MACE) during the index hospitalization and at 30 days, 6 months, and 1 year after the initial XIENCE V stent implantation.
The primary end point was MACE at 1 year, defined as the composite of death, myocardial infarction (MI), and target lesion revascularization (TLR). Q-wave MI was defined as evidence of new Q waves on the electrocardiogram. MI was defined as a total creatinine kinase increase of ≥2× the upper limit of normal and/or creatinine kinase (MB fraction) ≥20 ng/ml, together with symptoms and/or ischemic electrocardiographic changes. Hypercholesterolemia was defined as fasting cholesterol >250 mg/dl or the use of lipid-lowering therapy. Systemic hypertension was defined as blood pressure >140/90 mm Hg or the use of antihypertensive therapy. Renal impairment was defined as serum creatinine >1.2 mg/dl. Congestive heart failure was defined as evidence of fluid retention from cardiac causes before admission. Angiographic success was defined as postprocedural stenosis of <30% and Thrombolysis In Myocardial Infarction flow grade 3. TLR was defined as ischemia-driven percutaneous or surgical repeat intervention in the stent or within 5 mm proximal or distal to the stent. Stent thrombosis was defined in accordance with the Academic Research Consortium definitions as definite or probable stent thrombosis.
Statistical analysis was performed using SAS, version 8.2 (SAS Institute, Cary, North Carolina). Continuous variables and categorical variables are expressed as the mean ± SD and percentages, respectively. Analyses of the differences among the 3 DESs were performed using analysis of variance for continuous variables and the chi-square test or Fisher’s exact test for categorical variables. For variables with a 3-group p value <0.05, 2 subsequent pair-wise comparisons for XIENCE versus TAXUS and CYPHER were performed with Bonferroni’s correction for p value significance (p <0.025). After univariate analysis for baseline clinical and procedural characteristics, the following characteristics with p <0.1 were incorporated into the multivariate analysis to assess the independent association with MACE and stent thrombosis: stent type used, history of coronary artery disease, hypertension, left ventricular ejection fraction, diabetes mellitus, number of diseased vessels, left anterior descending artery disease, American College of Cardiology/American Heart Association lesion type classification, number of treated lesions per patient, maximum stent length and diameter, number of implanted stents, acute MI, and clopidogrel compliance. Clopidogrel compliance was expressed as a time-dependent covariate defined as the days to cessation for those patients who stopped their prescription. The stent type was entered as a categorical variable with XIENCE V as the reference. The results are presented as adjusted hazard ratios, with the 95% confidence intervals. Survival, MACE, and stent thrombosis rates ≤1 year were done using the Kaplan-Meier method, and differences in parameters were assessed using the log-rank test. p Values <0.05 were considered statistically significant.
Results
The data from 6,069 patients were analyzed. Of these patients, 3,423 (56%) received a CYPHER stent, 1,650 (27%) received a TAXUS stent, and 996 (17%) received a XIENCE V stent. The average age of the population was 65 ± 12 years, they were mostly men (65%), and 57% of the patients presented with acute MI or unstable angina pectoris ( Table 1 ). The baseline patient characteristics were generally comparable among the 3 groups ( Table 1 ). However, patients who received a XIENCE V stent had a significantly greater prevalence of cardiovascular risk factors, including diabetes, arterial hypertension, history of percutaneous coronary intervention, and coronary artery bypass surgery. The indication for percutaneous coronary intervention was acute MI or unstable angina in 63% of the XIENCE V patients compared to 55% of the CYPHER and 57% of the TAXUS patients.
| Variable | Overall (n = 6,069) | CYPHER (n = 3,423) | TAXUS (n = 1650) | XIENCE V (n = 996) | p Value |
|---|---|---|---|---|---|
| Age (years) | 65 ±12 | 65 ±12 | 65 ±12 | 64 ±11 | 0.09 |
| Men | 3,940 (65%) | 2,209 (65%) | 1,080 (66%) | 651 (65%) | 0.79 |
| European American | 4,175 (69%) | 2,414 (71%) | 1,112 (67%) | 649 (1,004) | 0.002 |
| African American | 1,434 (24%) | 731 (21%) | 414 (25%) | 289 (29%) | <0.001 |
| Diabetes mellitus | 2,126 (35%) | 1,166 (35%) | 560 (34%) | 400 (41%) | <0.001 |
| Chronic renal failure | 781 (13%) | 427 (13%) | 218 (13%) | 136 (14%) | 0.56 |
| Hypertension ⁎ | 5,173 (85%) | 2,862 (84%) | 1,416 (86%) | 895 (90%) | <0.001 |
| Hypercholesterolemia † | 5,334 (89%) | 3,003 (89%) | 1,443 (88%) | 888 (89%) | 0.62 |
| Current smoker | 1,172 (19%) | 646 (19%) | 325 (20%) | 201 (20%) | 0.59 |
| Family history of coronary artery disease | 3,046 (52%) | 1,749 (53%) | 818 (52%) | 479 (48%) | 0.02 |
| Previous myocardial infarction | 1,299 (23%) | 696 (22%) | 349 (22%) | 254 (26%) | 0.004 |
| Previous percutaneous coronary intervention | 1,762 (30%) | 929 (28%) | 443 (28%) | 390 (40%) | <0.001 |
| Previous coronary artery bypass grafting | 1,154 (19%) | 624 (18%) | 309 (19%) | 221 (22%) | 0.02 |
| Previous heart failure | 774 (13%) | 443 (14%) | 195 (12%) | 136 (14%) | 0.35 |
| Peripheral vascular disease | 916 (15%) | 507 (15%) | 263 (16%) | 146 (15%) | 0.52 |
| Procedure indication | |||||
| Myocardial infarction this admission | 741 (12%) | 435 (13%) | 195 (12%) | 111 (11%) | 0.36 |
| Unstable angina pectoris | 2,703 (45%) | 1,444 (42%) | 743 (45%) | 516 (52%) | <0.001 |
| Stable angina pectoris | 2,605 (43%) | 1,533 (45%) | 706 (43%) | 366 (37%) | <0.001 |
⁎ Blood pressure >140/90 mm Hg or the use of antihypertensive therapy.
† Fasting cholesterol >250 mg/dl or the use of lipid-lowering therapy.
The anatomic distribution of the treated vessels was generally comparable among the 3 groups, with overall rates of left main intervention of 1.8% and saphenous vein graft intervention of 4.7% ( Table 2 ). Patients treated with a XIENCE V stent had more complicated lesions as indicated by nearly twofold greater rates of American College of Cardiology/American Heart Association type C lesions compared to the other 2 stent groups. No clinically significant differences were found in the number of stents used per lesion, maximum stent diameter, or total stent length ( Table 2 ). The maximum troponin levels after the procedure were similar among the 3 groups.
| Variable | Overall (n = 6,069) | CYPHER (n = 3,423) | TAXUS (n = 1650) | XIENCE V (n = 996) | p Value |
|---|---|---|---|---|---|
| Coronary lesion location and characteristics | |||||
| Left anterior descending | 3,982 (40%) | 2,406 (43%) | 1,017 (36%) | 559 (39%) | <0.001 |
| Left circumflex | 2,261 (23%) | 1,260 (22%) | 645 (23%) | 356 (25%) | 0.19 |
| Right | 2,946 (30%) | 1,541 (27%) | 962 (34%) | 443 (31%) | <0.001 |
| Left main | 180 (1.8%) | 111 (2%) | 45 (1.6%) | 24 (1.7) | 0.44 |
| Saphenous vein graft | 459 (4.7%) | 285 (5.1%) | 116 (4.1%) | 58 (4%) | 0.08 |
| Type A lesion | 705 (7%) | 407 (8%) | 146 (5%) | 152 (11%) | <0.001 |
| Type B1 or B2 lesion | 6,455 (67%) | 3,893 (72%) | 1,931 (71%) | 631 (44%) | <0.001 |
| Type C lesion | 2,414 (25%) | 1,118 (21%) | 643 (24%) | 653 (46%) | <0.001 |
| Diameter stenosis (%) | 84 ± 11 | 85 ± 10 | 85 ± 10 | 81 ± 16 | <0.001 |
| Procedural data | |||||
| Treated lesions per patient | 1.6 ± 1.0 | 1.6 ± 1.3 | 1.6 ± 0.9 | 1.4 ± 0.7 | <0.001 |
| Number of stents | 1.5 ± 0.8 | 1.5 ± 0.8 | 1.6 ± 0.8 | 1.5 ± 0.7 | <0.001 |
| Maximum stent diameter per lesion (mm) | 3.1 ± 2.7 | 3.1 ± 2.5 | 3.1 ± 1.5 | 3.2 ± 4.4 | 0.74 |
| Total stent length per lesion (mm) | 19.9 ± 6.5 | 20.6 ± 6.8 | 19.5 ± 6.5 | 18.4 ± 5.3 | <0.001 |
| Glycoprotein IIb/IIIa inhibitors | 528 (8.7%) | 362 (10.6%) | 129 (7.9%) | 37 (37%) | <0.001 |
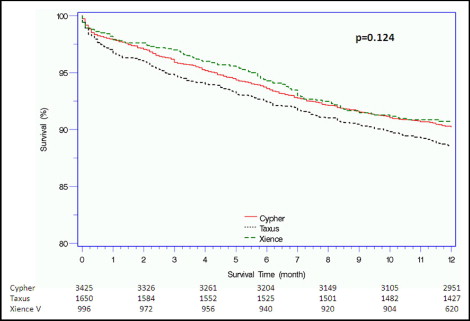
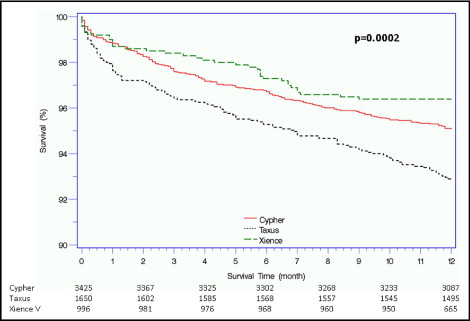
Complete 1-year follow-up data were available for 4,998 patients (82%; Table 3 ). The primary end point of MACE at 1 year occurred in 615 patients (10.2%). No significant differences were found among the groups in terms of MACE rates; however, patients who received a TAXUS stent tended to have slightly greater rates (11.5%) compared to the CYPHER and XIENCE V patients (9.8% and 9.3%, respectively; Figure 1 ). MACE were driven mainly by the 1-year all-cause mortality, which was lowest among patients who received XIENCE V (3.6%) compared to TAXUS (7.2%, p = 0.002, for XIENCE vs TAXUS) and CYPHER (4.9%, p = 0.72, for XIENCE vs CYPHER; Figure 2 ), and by TLR events, which were comparable among the 3 groups (p = 0.34). Cardiac mortality, both in- and out-of-hospital, tended to be greater among those who received a TAXUS stent. A multivariate model designed to assess the association among stent type, baseline and procedural characteristics, and MACE at 1 year showed that stent type did not have an independent effect on outcome. Left ventricular ejection fraction, diabetes mellitus, number of implanted stents, and maximum stent length were associated with adverse 1-year outcomes ( Table 4 ).
| Variable | Overall (n = 6,069) | CYPHER (n = 3,423) | TAXUS (n = 1650) | XIENCE (n = 996) | p Value |
|---|---|---|---|---|---|
| Major adverse cardiovascular events | 615 (10.2%) | 334 (9.8%) | 189 (11.5%) | 92 (9.3%) | 0.11 |
| All-cause mortality | 322 (5.3%) | 168 (4.9%) | 118 (7.2%) | 36 (3.6%) | <0.001 |
| Q-wave myocardial infarction | 16 (0.3%) | 6 (0.2%) | 8 (0.5%) | 2 (0.2%) | 0.14 |
| Target lesion revascularization | 305 (5.2%) | 175 (5.2%) | 73 (4.6%) | 57 (5.9%) | 0.34 |
| Cardiac mortality, in-hospital | 45 (0.7%) | 19 (0.6%) | 19 (1.2%) | 7 (0.7%) | 0.07 |
| Cardiac mortality, out of hospital | 68 (1.1%) | 26 (0.8%) | 31 (1.9%) | 11 (1.1%) | 0.002 |
| Non–Q-wave myocardial infarction | 78 (1.3%) | 44 (1.3%) | 21 (1.3%) | 13 (1.3%) | 1.0 |
| Target vessel revascularization | 460 (7.8%) | 272 (8.1%) | 112 (7.0%) | 76 (7.8%) | 0.41 |
| Stent thrombosis | 56 (0.9%) | 42 (1.2%) | 12 (0.7%) | 2 (0.2%) | 0.007 |