Significant variability in activated clotting time (ACT) measurement exists based on the type of point-of-care system used. We sought to determine the degree of agreement in ACT measurements by the Hemochron Response and the Hemochron Signature Elite Whole Blood coagulation systems and whether these 2 systems can be used interchangeably. We prospectively compared 126-paired samples in 77 patients undergoing percutaneous coronary intervention. ACT was measured for each sample using the Hemochron Response system with glass test tubes and the Hemochron Signature Elite system with low-range ACT cuvettes simultaneously. We used correlation and Bland–Altman analyses. Mean age of the study cohort was 67 ± 11 years, 49% were women, and 65% of measurements were made after systemic anticoagulation. There was a significant correlation between the Hemochron Response and Hemochron Signature Elite systems (r = 0.84, p <0.01). However, the mean bias for the ACT measurement was 9 seconds (95% confidence interval −69 to 86). In the therapeutic range of ACT measurements, the mean bias was 15 seconds (95% confidence interval −60 to 91). Thirty-three percent of total samples had >10% disagreement and 8% of samples had >20% disagreement in the ACTs measured with the Hemochron Response compared to the Hemochron Signature Elite. In conclusion, the Hemochron Response and Hemochron Signature Elite ACT values cannot be used interchangeably. Institutions using these 2 devices should be cognizant of this difference for ensuring patient safety.
Activated clotting time (ACT) measurement can vary based on the type of point-of-care system used. Currently, the Hemochron system offers 2 different systems for assessing ACT: the Hemochron Response and the Hemochron Signature Elite. The newer Hemochron Signature Elite system measures ACT based on electronic optical detection of clot formation versus the mechanical detection of clot formation in the traditional Hemochron Response system. In addition, the Hemochron Signature Elite system is designed for measuring ACT with low to moderate doses of heparin (≤2.5 U/ml), whereas the Hemochron Response system measures ACT with higher doses of heparin. It is unclear whether there is any difference in ACT values measured using the Hemochron Response and Hemochron Signature Elite systems and whether they can be used interchangeably. In anecdotal experience, we observed a difference in the values of ACT determined by these 2 techniques, with a potential to adversely affect the management of anticoagulation during a percutaneous coronary intervention (PCI) procedure and while titrating anticoagulation dose on the hospital floor. Accordingly, a quality control initiative was undertaken and we performed a prospective observational study to compare ACT values measured simultaneously using the Hemochron Response and Hemochron Signature Elite systems in whole-blood samples obtained during PCI.
Methods
We collected 126 paired whole-blood samples from 77 patients who were referred to our cardiac catheterization laboratory for PCI from March 2010 through January 2011. All patients received an unfractionated heparin (UFH) dose based on their weight to achieve a therapeutic ACT. A therapeutic ACT was defined as >200 seconds based on the Hemochron Response ACT value. ACT was measured before (baseline) and after administration of UFH. All patients gave written informed consent for participation in the study, and the human institutional review board at the University of Chicago approved this study.
Five milliliters of blood was collected from each patient from the arterial or venous sheath using unheparinized syringes after elimination of the first 5 ml of blood. ACT was measured simultaneously using the Hemochron Response and Hemochron Signature Elite analyzers without any delay. The Hemochron Response clot detection module contains 2 test wells into which disposable unitized coagulation test tubes can be inserted. It uses disposable flip-top nonevacuated glass test tubes containing a precision magnet for clot detection and Celite-based reagents (diatomaceous earth; FTCA510, International Technidyne Corp, Edison, New Jersey), which activates the coagulation process. Immediately after the sample is added to the test tube, the test tube is agitated and placed into the test well. There, it is automatically rotated at a controlled speed and incubated at 37 ± 1.0°C. When a fibrin clot begins to form, it displaces the magnet in the test tube. Two magnetic detectors located in the test well continuously monitor the precise magnet position. When a specific displacement of the magnet occurs, the elapsed time from the beginning of the test to the clot end point is displayed as the coagulation time (Hemochron Response package insert).
The Hemochron Signature Elite system measures whole-blood clotting times using a microprocessor-based analyzer and disposable single-use cuvettes (ACT-LR) containing a test chamber preloaded with test reagents. The operator inserts a cuvette for the test into the instrument. After the cuvette has warmed to 37 ± 1.0°C, the instrument beeps, signaling the operator that a blood sample can be added. A drop of blood is placed in the sample well of the cuvette. The instrument measures the required volume of blood (15 μl) and automatically moves it into the cuvette test channel, where it is mixed with the ACT-LR test reagents (Celite, potato dextrin, stabilizer, and buffer) in the reaction chamber. After mixing with the reagent, the sample is moved back and forth at a predetermined rate within the test channel and monitored for clot formation. The test channel is maintained at 37 ± 1.0°C during the test. The rate of movement of the sample is monitored by a series of light-emitting diode optical detectors that are aligned with the test channel. When the blood clots, the flow of the blood sample within the test channel is impeded, decreasing its rate of flow between the optical detectors. This decrease in flow below a predetermined value signals to the instrument that a clot has formed. An internal timer measures the elapsed time from the start of the test to clot formation. The actual clotting time for each test is then extrapolated using a predetermined validated conversion formula and ACT-LR results are displayed as a Celite-equivalent ACT (Hemochron Signature Elite package insert). The Hemochron Response and Hemochron Signature Elite analyzers were calibrated daily according to the manufacturer’s instructions.
Continuous variables were expressed as mean ± SD and categorical variables were expressed as frequency and percentage. Difference in mean ACT measured by the 2 systems was compared using paired t test. Correlation coefficients between the 2 ACT systems were determined using the Pearson correlation method. Because a high correlation does not always reflect a better agreement, to truly measure the agreement between the 2 systems, we used Bland–Altman analyses, which plot the difference between 2 techniques against their mean. All analyses were done separately for ACT measurements performed before (baseline) and after heparin administration (therapeutic). All statistical analyses were performed using STATA 10 (STATA Corp., College Station, Texas).
Results
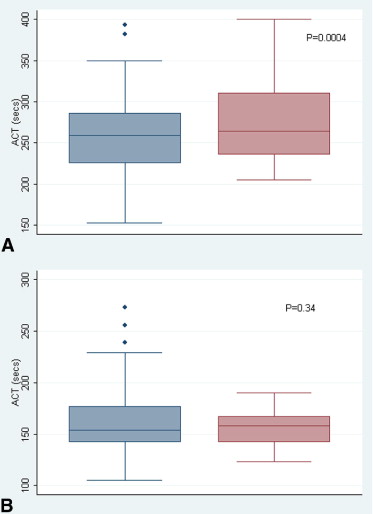
Mean age of the study cohort was 67 ± 11 years and 49% were women. Of the 126 paired blood samples, 45 (35%) were drawn at baseline and 83 (65%) were drawn after administration of UFH. The mean Hemochron Signature Elite ACT (278 ± 54 seconds, range 205 to 400) was higher than the mean Hemochron Response ACT (262 ± 47 seconds, range 153 to 393, p = 0.0004) in the therapeutic range of ACT (>200 seconds based on Hemochron Response results; Figure 1 ) , but there was no difference in mean ACT between the 2 systems (Hemochron Signature Elite ACT 157 ± 18 seconds, range 123 to 190; Hemochron Response ACT 163 ± 38 seconds, range 105 to 273, p = 0.34, respectively) in the subtherapeutic range of ACT (<200 seconds; Figure 1 ). The overall Pearson correlation coefficient (including subtherapeutic and therapeutic ACT values) between the 2 systems was 0.84 and the correlation coefficient in the therapeutic range of ACT was 0.72 ( Figure 2 ).

Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


