Inadequate first procedure (sampling error)
Restaging of lung cancer after induction therapy
Inflammatory mediastinal diseases
Mediastinitis
Recurrent lung cancer
Metachronous second primary lung cancer
Lung cancer occurring after unrelated disease (e.g. sarcoidosis, lymphoma, tuberculosis)
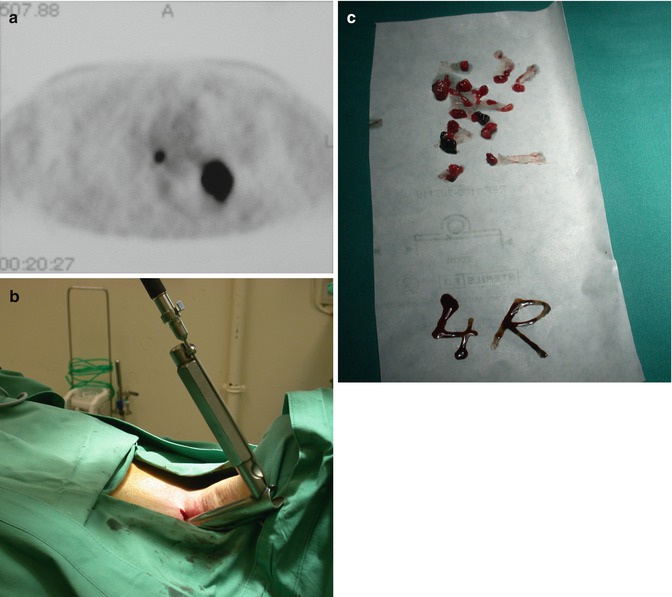
Fig. 4.1
A 60-year-old male patient who underwent lobectomy of left upper lobe in 1996. During follow-up recurrent lung cancer was detected at the left hilum. Positron emission tomography was positive at the left hilum but also showed a hot spot in the right lower paratracheal region (a). Remediastinoscopy was performed with introduction of the mediastinoscope at the left paratracheal gutter (b). Large biopsies of lower paratracheal lymph node station 4R could be taken which proved to be negative (c). Subsequently, a completion pneumonectomy was performed
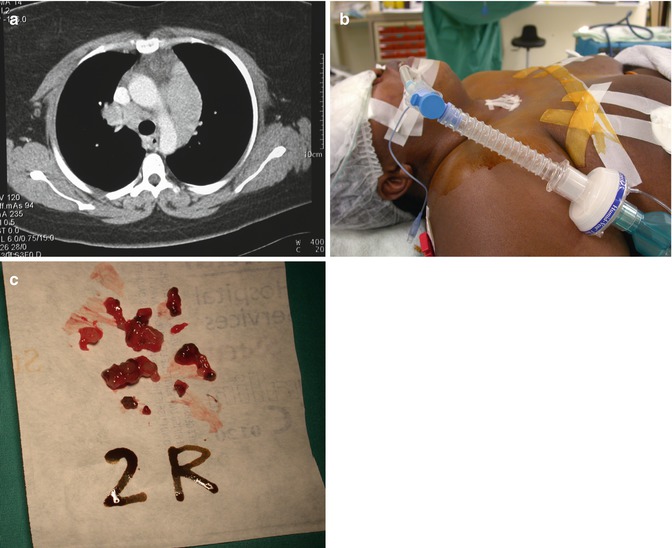
Fig. 4.2
This 32-year-old female patient underwent a cervical mediastinoscopy in 2002 in Sweden which only showed reactive lymph nodes. Because of increasing dyspnoea and progressive mediastinal lymphadenopathy on chest computed tomography (a), a repeat mediastinoscopy was performed. Due to extreme obesity, straps had to be placed on both breasts to gain access to the previous incision (b). Large biopsies were obtained from upper paratracheal station 2R (c). Definitive diagnosis of Castleman’s disease was made
4.4 Contraindications
Contraindications for remediastinoscopy are not different from those of a first procedure. Caution should be exerted in case of a large substernal goitre, severe coagulation abnormalities, Bechterew’s disease with spinal rigidity, definitive tracheostomy, pronounced superior vena cava syndrome and history of high-dose mediastinal radiotherapy, which are considered relative or absolute contraindications to a repeat procedure.
4.5 Technique
When performing a remediastinoscopy, the same incision as for the initial procedure can be used. Due to scar tissue formation, sharp dissection is often necessary to go through the fibrotic strap muscles in order to reach the left paratracheal plane. Usually, the brachiocephalic trunk is densely adherent to the trachea, making dissection in this area a major danger point. So, we advocate to bluntly create a tunnel on the left paratracheal side until the aortic arch is reached [10]. The mediastinoscope is introduced in this tunnel (Fig. 4.1b). To perform a remediastinoscopy, we prefer to use a classical mediastinoscope due to its small size allowing easy manoeuvrability in a constrained space. Underneath the aortic arch, it is fairly easy to cross to the right paratracheal space (Fig. 4.2c) and reach the lower paratracheal lymph node station 4R. Dissection is then continued in the pretracheal plane until subcarinal lymph node station 7 is reached. For this location it is important to stay on the posterior side of the right pulmonary artery. Subsequently, the fascia behind the aortic arch is opened to reach the pretracheal lymph nodes, formerly station 3, presently 4R or 2R. From this point it is possible to continue dissection in a more cranial way to reach the higher right paratracheal lymph nodes belonging to station 2R. In most instances, the brachiocephalic trunk is not dissected from the trachea to avoid injury to this vessel.
First of all, biopsies of the initially involved lymph node stations are carefully taken to evaluate response as progressive disease is unusual after induction therapy [10]. We were always able to reach the initially involved lymph node stations. Due to the sometimes dense adhesions or fibrosis, bleeding may occur, but this can usually be controlled by packing or electrocoagulation. If blood loss is severe, a thoracotomy or sternotomy may be indicated depending on the origin of haemorrhage. In principle no drain is inserted after remediastinoscopy as is the case for a primary mediastinoscopy in our centre.
4.6 Mediastinal Restaging in Lung Cancer
Mediastinal downstaging after induction therapy for locally advanced stage III non-small cell lung cancer is an important prognostic factor for long-term survival. Patients with persisting mediastinal involvement have a poor prognosis and will not benefit from surgical resection [11–13].
In the prospective, multicentre EORTC 08941 trial, patients with proven stage IIIA-N2 non-small cell lung cancer were randomised after a response to induction chemotherapy between surgery and radiotherapy [12]. Overall and progression-free survival were not significantly different between both arms. However, patients with downstaging to N0 or N1 disease had a far better prognosis. Similar results were observed in the INT 0139 trial randomising patients with proven stage IIIA-N2 non-small cell lung cancer between a full course of chemoradiotherapy and induction chemoradiation followed by surgery [13]. So, precise restaging after induction chemo- or chemoradiotherapy is of utmost importance to determine subsequent treatment and prognosis.
Different restaging methods are currently available, and they can be subdivided into noninvasive, minimally invasive and invasive methods (Table 4.2).
Table 4.2
Techniques for mediastinal restaging
Noninvasive |
Computed tomography (CT) |
Magnetic resonance imaging (MRI) |
Positron emission tomography (PET) |
Integrated PET-CT |
Minimally invasive |
Transthoracic fine-needle aspiration biopsy (FNAB) |
Transbronchial needle aspiration (TBNA) |
Endobronchial ultrasound with FNAB (EBUS) |
Endoscopic oesophageal ultrasound with FNAB (EUS) |
Invasive |
Repeat, second or remediastinoscopy (reMS) |
Video-assisted thoracic surgery (VATS) |
Noninvasive imaging techniques are not accurate enough for mediastinal restaging. PET provides additional metabolic information, but there are conflicting data regarding its accuracy in restaging. Moreover, PET has been shown to be more accurate in predicting the T factor than the N status [14]. Integrated PET-CT combining precise anatomical and functional information seems more accurate for mediastinal restaging. In a prospective study of 93 patients who were restaged by chest CT and integrated PET-CT after induction chemoradiotherapy, repeat PET-CT was found to be more accurate than CT alone for all pathological stages [15]. However, there were 20 % false-negative and 25 % false-positive cases. So, in case of suspicion of residual mediastinal disease, nodal biopsies are still required [15].
Minimally invasive techniques are promising staging modalities and are increasingly used for lung cancer staging and also restaging (Table 4.3). However, false-negative rates between 20 and 58 % have been reported [15–21].
Table 4.3
Restaging with PET-CT, EUS and EBUS after induction therapy
Author, year | Ref. | Technique | n | Sensitivity (%) | Specificity (%) | Accuracy (%) |
|---|---|---|---|---|---|---|
Cerfolio et al., 2006 | [15] | PET-CT | 93 | 62 | 88 | 79 |
De Leyn et al., 2006 | [16] | PET-CT | 30 | 77 | 92 | 83 |
Herth et al., 2008 | [17] | EBUS | 124 | 76 | 100 | 77 |
Negative predictive | ||||||
Value: 20 % | ||||||
Stigt et al., 2009 | [18] | EUS | 25 | 92 | 100 | 92 |
PET-CT | 83 | 31 | 56 | |||
Szlubowski et al., 2010 | [19] | EBUS | 61 | 67 | 86 | 80 |
von Bartheld et al., 2011 | [20] | EUS | 58 | 44 | NR | 60 |
False-negative rate: 58 % | ||||||
Zieliński et al., 2013 | [21] | EBUS 32 pts | 88 | 64.3 | 100 | NR |
EUS 6 pts | Negative predictive | |||||
CUS 50 pts | Value: 82.1 % |
4.7 Results of Remediastinoscopy
Results of recent series of remediastinoscopy after induction therapy are summarised in Table 4.4 [9, 16, 22–26]. Repeat mediastinoscopy offers the advantage of providing pathological evidence of response after induction therapy, and in this way, it remains a valuable tool to select patients for surgical resection [5]. Survival clearly depends on the findings of remediastinoscopy, patients with persisting mediastinal involvement having a poor prognosis compared to those with negative remediastinoscopy [24]. In a combined series of 104 patients, nodal status was the only significant factor related to survival in multivariate analysis [25].
Table 4.4




Results of remediastinoscopy after induction therapy
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


