Osteoprotegerin (OPG) is a member of the tumor necrosis factor receptor superfamily and is known to be among the mediators of the calcification process that has been shown to increase in patients with aortic stenosis (AS). The aim of this study was to characterize the association of OPG with left ventricular (LV) function and remodeling and to evaluate the significance of preoperative OPG on long-term outcome in terms of survival and symptomatic improvement in 124 patients with severe AS scheduled for aortic valve replacement (AVR). Patients were divided according to tertiles of preoperative OPG. Preoperative OPG was associated with age, EuroSCORE, and preoperative functional capacity. Despite similar ejection fraction and diastolic function among groups, longitudinal LV systolic function consistently decreased and markers of filling pressure increased across groups. During median follow-up of 4 years, 41 patients died of a presumed cardiovascular cause or remained in New York Heart Association functional class III or IV. The risk of a poor postoperative outcome after AVR increased with increasing OPG tertiles (15% vs 33% vs 51%, p = 0.002). In a multivariate model containing age, ejection fraction, N-terminal pro-brain natriuretic peptide and left atrial volume index, OPG was still significantly associated with postoperative outcome. In addition, OPG levels associated with cardiovascular mortality during follow-up. In conclusion, OPG is associated with LV and left atrial remodeling in patients with symptomatic severe AS undergoing AVR. Moreover, plasma OPG is associated with long-term postoperative outcome and may identify patients with poor symptomatic improvement after surgery.
Aortic stenosis (AS) is a progressive disease in which active bone formation can be seen in the cusps, compromising valve-opening and causing left ventricular (LV) pressure overload. Osteoprotegerin (OPG) is a member of the tumor necrosis factor receptor superfamily and is among the mediators of the calcification process. Increased OPG levels have been associated with traditional cardiovascular risk factors, such as duration of diabetes and severity of peripheral vascular disease, and will also increase with age. It is well established that OPG levels are increased in heart failure and increase with disease severity. In AS, OPG levels are elevated and correlate with pulmonary capillary wedge pressure independent of coronary arteriosclerosis. In addition, a recent study demonstrated that OPG levels are associated with clinical outcome in severe AS with preserved LV ejection fraction. Current guidelines put heavy emphasis on patients’ symptoms for the timing of aortic valve replacement (AVR), without standardizing under which conditions symptoms should be present. This is based on historic data suggesting an exponential increase in mortality with the development of symptoms. However, with increasing age and comorbidity among patients with AS considered for valve replacement, the need for more quantitative indices of the consequence of AS to select patients who will benefit from valve replacement is growing. The purposes of this cross-sectional study were thus to characterize the association of OPG with longitudinal LV function and LV remodeling parameters and to evaluate the significance of preoperative OPG on long-term cardiovascular mortality and symptom improvement after AVR in patients with AS.
Methods
The present study is a substudy of a prospective single-center randomized study to evaluate the effect of candesartan on top of conventional treatment on reverse remodeling in consecutive patients undergoing AVR for symptomatic AS. The study was registered with the National Board of Health and the Danish Data Protection Agency and was approved by the local ethical committee. All patients gave written informed consent. The study is registered at ClinicalTrials.gov (Identifier: NCT00294775 ). The study design and effect of candesartan on regression of LV hypertrophy has been previously published. In brief, patients aged >18 years with symptomatic severe AS (estimated aortic valve area <1 cm 2 ) planned for AVR at Odense University Hospital, Denmark, from February 2006 to April 2008 were enrolled. Patients with LV ejection fraction <40%, serum level of creatinine >220 μmol/L, previous aortic valve surgery, planned additional valve repair or replacement, infective endocarditis, predominant aortic valve regurgitation, or ongoing treatment with an angiotensin converting enzyme inhibitor or an angiotensin receptor blocker were excluded.
Blood samples were collected the day before AVR after the patients had been resting for at least 30 minutes. Samples were collected in ethylenediamine tetra-acetic acid tubes. These were then centrifuged and plasma samples stored at −80°C for later analysis. N-terminal pro-brain natriuretic peptide (NT-proBNP) was determined using an ELECSYS proBNP immunoassay (Roche Diagnostics GmbH, Mannheim, Germany). Plasma OPG concentrations were measured in ethylenediamine tetra-acetic acid-plasma using an immunoassay based on commercially available sandwich enzyme-linked immunosorbent assay (R&D Systems, Minneapolis, Minnesota) with specific antibodies against human OPG and europium-labeled streptavidin for detection. Bound europium was measured by time-resolved fluorometric detection using an Autodelfia Instrument from Perkin Elmer/Wallac (Turku, Finland). The analytical range was 62.5 to 20,000 ng/L with an intraassay imprecision of 4% and an interassay imprecision of 7%. Patients were divided in 3 groups according to OPG tertiles.
All echocardiograms were performed the day before surgery by the same operator on a GE Vivid 5 ultrasound machine (GE Medical System, Horten, Norway). All echocardiograms were digitally stored and later analyzed completely blinded for all clinical and survival data.
Aortic valve area was estimated with quantitative Doppler using the continuity equation. The diameter of the LV outflow tract was measured 5 mm below the annulus from a zoomed image of the LV outflow tract obtained in the parasternal long axis view. Peak flow velocity across the valve was determined by Doppler interrogation from the apical window and multiple other echocardiographic windows. The highest peak velocity that could be obtained by placing the continuous-wave Doppler cursor as parallel as possible with the flow across the valve was selected. Peak transvalvular gradient was estimated using the modified Bernoulli equation. Finally, the peak systolic flow velocity in the outflow tract was assessed with pulsed-wave Doppler. LV ejection fraction was estimated using Simpson’s biplane method.
LV mass was estimated according to the joint recommendations of the American Society of Echocardiography and European Association of Echocardiography using the Devereux formula. LV wall thickness and dimensions were estimated from the average of 3 consecutive 2-dimensional images obtained in the parasternal long axis view according to guidelines. Left atrial (LA) volume was measured in LV end systole in the frame preceding mitral valve opening. The volume was measured using the biplane area length method and corrected for body surface area.
Mitral inflow was assessed in the apical 4-chamber view using pulsed-wave Doppler with the sample volume placed at the tips of the mitral leaflets during diastole. From the mitral inflow profile, the E- and A-wave peak velocities and deceleration time were measured. Doppler tissue imaging of the mitral annulus was obtained from the apical 4-chamber view, using a sample volume placed in the septal mitral valve annulus. The e′ velocity was estimated and the E/e′ ratio derived. For all Doppler recordings, a horizontal sweep of 100 mm/s was used. For patients in sinus rhythm, the average of 5 consecutive beats were measured; for patients with atrial fibrillation, 10 beats were averaged.
Global longitudinal strain was analyzed using EchoPAC PC 08 (GE Medical Systems, Horten, Norway) speckle tracking two-dimensional software. Global strain was determined as the magnitude of strain at the aortic valve closure, and systolic strain rate (SR S ) was determined as the maximal negative SR value during the ejection phase. Both parameters were assessed in all 3 apical planes, and the mean values (GLS mean, SRs mean ) were calculated. Frame rate was kept as high as possible with a minimum frame rate of 70/s.
A clinical examination assessing symptoms, and signs of heart failure including a 6-minute walk test was performed before surgery in all patients as part of this study. By July 2011, data on outcomes were collected from the Danish Personnel Register (survival status) and from discharge notes available in the Danish admission registry. In case of ambiguous information, local hospitals were contacted and detailed patient charts were reviewed. In addition, we were able to contact 82 patients of the 95 survivors by telephone during October 2011, and functional status/quality of life was assessed using New York Heart Association (NYHA) functional class and the Duke Activity Status Index self-questionnaire. The 12-item scale ranging from 0 (worst) to 58.2 (best) evaluates the ability to perform activities of daily living. The main end point for this study was a poor postoperative outcome, defined as NYHA functional class III/IV by October 2011, or cardiac death during follow-up. A secondary end point was cardiovascular mortality. End points were ascertained by 1 of the investigators who was as blinded to all echocardiographic measurements and OPG levels.
Data are presented as mean ± standard deviation or number and percentages. Differences between groups were tested using analysis of variance; categorical variables were tested by Fisher’s exact test. In case of significant difference between groups, paired comparisons were performed using Tukey’s range test. Due to a non-Gaussian distribution, OPG and NT-proBNP are presented as median and interquartile range. Echocardiographic, demographic, and laboratory data were assessed for association with a poor postoperative outcome by univariate and multivariate analyses using logistic regression. OPG was tested in several multivariable models including one containing the predefined parameters age, ejection fraction, LA volume index, and NT-proBNP. Interaction terms were included in the multiple regression analysis to examine the potential modulating effect of clinically relevant covariates on postoperative outcome. In addition to odds ratios, adjusted odds ratios for 1 standard deviation were calculated, dividing the variable by the standard deviation of the measure. Comparison of each method’s predictive capability was performed by comparing the C-statistic derived from the area under the receiver operating characteristic curves using the generalized U-statistic as proposed by DeLong et al.
Cardiovascular mortality was estimated using Cox proportional hazard models; because of limited events, we only performed univariate analyses. The assumptions (proportional hazard assumption, linearity of continuous variables, and lack of interaction) were tested and found valid. A p value <0.05 was considered significant. STATA/SE 9.0 (StataCorp, College Station, Texas) software was used for statistical analysis.
Results
OPG in the complete cohort was a median of 1,800 ng/L (1,402 to 2,325) and correlated positively with age (r = 0.48, p <0.0001) and EuroSCORE (r = 0.48, p <0.0001). Preoperative New York Heart Association class was similar among groups; however, functional capacity measured as the 6-minute walk test was significantly reduced with increasing OPG (392 ± 106 vs 332 ± 124 vs 295 ± 129 meters, p = 0.005) ( Table 1 ). NT-proBNP and serum level of creatinine increased with increasing tertile of OPG. Use of a mechanical prosthesis was more common among patients with low OPG levels.
| Variable | OPG Tertile | p Value | ||
|---|---|---|---|---|
| Lowest (n = 41) | Middle (n = 42) | Highest (n = 41) | ||
| Age (yrs) | 67 ± 10 | 73 ± 7 | 77 ± 7 | <0.0001 |
| Men | 31 (76%) | 27 (64%) | 20 (49%) | 0.04 |
| Diabetes mellitus | 5 (12%) | 4 (10%) | 10 (24%) | 0.14 |
| Coronary heart disease | 7 (17%) | 10 (24%) | 6 (15%) | 0.54 |
| Atrial fibrillation | 4 (10%) | 4 (10%) | 11 (27%) | 0.04 |
| Symptoms | ||||
| NYHA class 1/2/3 | 11/20/10 | 5/25/12 | 7/19/15 | 0.42 |
| 6-minute walk test (min) | 392 ± 106 | 332 ± 124 | 295 ± 129 | 0.005 |
| Treatment | ||||
| Diuretics | 10 (24%) | 14 (33%) | 20 (49%) | 0.07 |
| β blockers | 8 (20%) | 10 (24%) | 10 (24%) | 0.84 |
| Calcium antagonist | 7 (17%) | 10 (24%) | 8 (20%) | 0.74 |
| Candesartan | 19 (46%) | 24 (57%) | 19 (46%) | 0.52 |
| Systolic blood pressure (mm Hg) | 145 ± 18 | 146 ± 20 | 146 ± 23 | 0.95 |
| EuroSCORE | 4.6 ± 1.9 | 5.9 ± 1.3 | 6.8 ± 2.0 | <0.0001 |
| Logistic EuroSCORE | 3.8 ± 3.0 | 5.1 ± 2.1 | 7.6 ± 5.4 | 0.0001 |
| Operation data | ||||
| Concomitant CABG | 9 (22%) | 15 (36%) | 13 (32%) | 0.37 |
| Mechanical prosthesis | 11 (27%) | 6 (14%) | 2 (5%) | 0.02 |
| Valve size | 24.6 ± 1.9 | 24.7 ± 2.9 | 23 ± 2.8 | 0.20 |
| Bioprosthesis | 30 (78%) | 36 (86%) | 39 (95%) | 0.02 |
| Valve size | 22.1 ± 6.4 | 22.8 ± 4.3 | 22.8 ± 4.2 | 0.67 |
| Serum creatinine (μmol/L) | 96 ± 16 | 97 ± 17 | 106 ± 29 | 0.05 |
| NTproBNP, median (IQR), pmol/L | 255 (149–675) | 425 (179–1,336) | 979 (355–2,546) | <0.0001 |
| OPG, median (IQR), ng/L | 1,279 (1,033–1,400) | 1,800 (1,684–1,943) | 2,519 (2,351–3,013) | <0.0001 |
There was no difference in severity of AS among groups ( Table 2 ). Increased OPG was associated with increased LV mass index. Despite a similar LV ejection fraction and diastolic function between groups, longitudinal LV systolic function consistently decreased across groups and markers of LV filling pressure increased across groups.
| Variable | OPG Tertile | p Value | ||
|---|---|---|---|---|
| Lowest (n = 41) | Middle (n = 42) | Highest (n = 41) | ||
| Aortic valve area (cm 2 ) | 0.82 ± 0.25 | 0.82 ± 0.26 | 0.80 ± 0.28 | 0.87 |
| Aortic velocity (m/s) | 3.9 ± 0.7 | 3.8 ± 0.7 | 4.0 ± 0.8 | 0.31 |
| Left ventricular mass index (g/m 2 ) | 120 ± 37 | 130 ± 42 | 142 ± 41 | 0.04 |
| Relative wall thickness | 59 ± 16 | 58 ± 14 | 62 ± 12 | 0.34 |
| Left atrial volume index (ml/m 2 ) | 43 ± 15 | 48 ± 18 | 56 ± 20 | 0.003 |
| Left ventricular end diastolic volume (ml) | 100 ± 32 | 117 ± 36 | 110 ± 33 | 0.09 |
| Left ventricular ejection fraction (%) | 55 ± 8 | 54 ± 8 | 54 ± 7 | 0.71 |
| Global strain (%) | −16.6 ± 3.2 | −15.4 ± 3.6 | −14.4 ± 4.1 | 0.03 |
| Strain rate (s −1 ) | −0.91 ± 0.15 | −0.84 ± 0.24 | −0.76 ± 0.21 | 0.004 |
| S′ average (cm/s) | 6.4 ± 1.1 | 6.4 ± 1.2 | 5.5 ± 1.5 | 0.002 |
| E-wave (m/s) | 0.7 ± 0.2 | 0.8 ± 0.2 | 0.8 ± 0.2 | 0.314 |
| A-wave (m/s) | 0.8 ± 0.3 | 1.0 ± 0.3 | 1.0 ± 0.3 | 0.02 |
| Deceleration time (ms) | 202 ± 56 | 201 ± 60 | 192 ± 63 | 0.68 |
| E/e′ sep | 12.5 ± 4.3 | 15.3 ± 4.5 | 16.8 ± 6.2 | 0.001 |
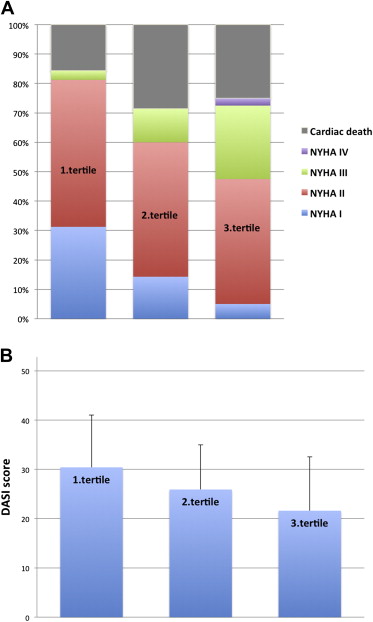
The mean follow-up in the entire population was 3.8 ± 1.5 years (median 4.0 years). Survival status was available for all patients. Overall, there were 29 deaths, 7 in the first tertile, 12 in the second, and 10 in the third. The cause of death was noncardiac in 4 patients (2 cancer, 1 infectious disease, 1 subarachnoid hemorrhage) and presumed cardiac in 25 (15 sudden cardiac death, 7 postoperative death, 2 heart failure, 1 aortic aneurism). Of the 95 survivors as of October 2011, estimation of functional status was possible in 82 patients. Duke Activity Status Index score was significantly reduced (30.4 ± 10.6 vs 25.9 ± 9.1 vs 21.6 ± 10.9, p = 0.008; Figure 1 and Table 3 ), and a greater proportion of patients were at NYHA functional class III or greater (4% vs 16% vs 37%, p = 0.002). As a consequence, patients with poor outcome after AVR increased with increasing tertile of OPG (15% vs 33% vs 51%, p = 0.002; Table 3 ).