Antibiotic chemotherapeutic agents
Bleomycin, Mitomycin C
Alkylating agents
Busulfan, Cyclophosphamide, Chlorambucil, Melphalan, Ifosfamide, Procarbazine
Antimetabolites
Methotrexate, 6-mercaptopurine, Cytosine arabinoside, Fludarabine
Nitrosamines
Bischloroethyl nitrosourea (BCNU), Chloroethyl cyclohexyl nitrosourea (CCNU), Methyl-CCNU
Tubulin-acting agents
Vinblastine, Etoposide
Other chemotherapeutic agents
All-trans retinoic-acid (ATRA), Imatinib mesylate, Dasatinib, Bortezomib
Immunomodulatory agents
Interferons, anti-Interleukin-2, TNF alpha inhibitors, Sirolimus, Temsirolimus
Monoclonal antibodies
Rituximab, Gemtuzumab ozogamicin, Alemtuzumab
Miscellaneous
Blood transfusion, GM-CSF, G-CSF
Different other “immunological” causes of pneumonitis may occur in the course of several hematological malignancies (Table 32.2). For example, sarcoid-like granulomatosis may be associated with lymphoma, eosinophilic pneumonia may occur in the course of a myelodysplastic syndrome and pulmonary graft-versus host disease (GVHD) may be a complication following allogeneic HSCT. The medical history of the patient, including the clinical history of lung involvement and the type of underlying malignancy, the lung CT scan and the bronchoalveolar lavage (BAL), should be used when making a diagnosis. A lung biopsy, when possible, would rule out infection and would reinforce or even confirm a diagnostic hypothesis. However, it is rarely performed in this context because it is associated with significant mortality and morbidity and results in a definitive diagnosis in only 60 % of cases [3]. Furthermore, new biological tools including polymerase chain reaction for many pathogens and antigen for Aspergillus used on both respiratory samples and sera greatly help to diagnose an infection [4]. Finally, the overall diagnostic approach of lung infiltrates in patients treated for a hematological malignancy must take into account the possible overlapping of different infectious and/or noninfectious causes.
Table 32.2
Nonspecific noninfectious pulmonary complications reported in hematological malignancies
Hematological malignancies | Pulmonary complications |
|---|---|
Acute leukemia | Organizing pneumonia [60] |
Sweet’s syndrome [61] | |
Alveolar proteinosis [62] | |
Lymphoma/chronic lymphocytic leukemia | |
Langerhans histiocytosis [65] | |
Myeloma | Amyloidosis [68] |
Venous thromboembolism [69] | |
Waldenstöm’s macroglobulinemia | Intra-alveolar hemorrhage [70] |
Pulmonary edema [70] | |
Lung cancer [71] | |
Myelodysplastic/myeloproliferative disorders | Extramedullary hematopoiesis [72] |
Sweet’s syndrome [72] | |
Diffuse infiltrative lung disease in the context of autoimmune disorders [72] | |
Eosinophilic pneumonia [72] | |
Alveolar proteinosis [72] | |
Organizing pneumonia [72] | |
Pulmonary hypertension [73] |
Pulmonary Manifestations of Allogeneic Hematopoietic Stem Cell Transplantation
Allogeneic HSCT is used as a curative treatment in various tumoral and non-tumoral diseases. The patient’s abnormal hematopoietic tissue is replaced with healthy stem cells.
The transplanted stem cells can be derived from bone marrow, peripheral blood or umbilical cord blood harvested from a related donor or a compatible unrelated donor. Before the transplantation procedure is performed, the host is prepared with a special conditioning regimen that usually involves fractionated total body irradiation and cytotoxic chemotherapy to prevent graft rejection and to eradicate any residual tumor cells. Until recently, the conditioning regimen was “myeloablative,” leaving the patient severely neutropenic for several weeks. Over the past few years, nonmyeloablative transplants have been developed based on the graft-versus leukemia/lymphoma principle, thereby eradicating residual tumor cells. The conditioning regimen for these transplants is attenuated, depressing only the host immune response and thus limiting the neutropenic period to just a few days. This means that allogeneic HSCT can now be extended to older and frailer patients with comorbidities who have had long-term treatment in the past, which has increased the number of allogeneic HSCT procedures performed. The major complications that occur after allogeneic HSCT are infections or the consequences of the immune reactions of GVHD, which can be either acute or chronic depending on the clinical features [5]. For unknown reasons, unlike the skin, gastrointestinal tract and liver, the lungs have not been identified as a target of acute GVHD in humans. In contrast, lung involvement is common in chronic GVHD that may be either restricted to a single organ or tissue or widespread. Chronic GVHD is characterized by tissue destruction resulting in fibrosis and is associated with a process in which donor T cells recognize peptides presented by the major histocompatibility complex on antigen-presenting cells of the host. GVHD targets epithelial cells of various organs with an incidence that depends on various factors and most commonly occurs during unrelated transplantations and peripheral stem cell transplantations. Treatment of GVHD requires the maintenance or enhancement of immunosuppressive therapy. The prognosis of GVHD is primarily related to the severity of the initial response to corticosteroids. The occurrence of chronic GVHD affects the morbidity, mortality and the quality of life of patients [6].
Chronic Pulmonary Graft Versus Host Disease
Chronic lung GVHD has been identified, the diagnosis of which is based on histopathological observations performed during lung biopsies showing bronchiolitis obliterans (BO). Although BO is the only condition attributed to pulmonary GVHD [5], other clinico-histological lung conditions are known to be associated with GVHD, such as organizing pneumonia (OP, formerly named bronchiolitis obliterans organizing pneumonia, BOOP) [5, 7]. Other noninfectious diffuse infiltrative lung diseases have been described and may also be associated with GVHD [8]. Although less frequent than BO, these diseases are often ignored, and their incidences may be underestimated.
Bronchiolitis Obliterans
Bronchiolitis obliterans (BO) is the main non-infectious late pulmonary complication in allogeneic HSCT recipients. This serious and potentially fatal complication typically develops in the first 2 years following transplant but also can occur several years later [9, 10]. The incidence of BO is difficult to assess; according to several retrospective studies, it varies from 2 to 26 % [11–16]. This disparity is mainly due to the lack of consensus regarding the diagnostic criteria. The most recent study, which was based on the largest number of patients, reported a prevalence of 5.5 % [15]. The incidence increased to 14 % in the subpopulation of patients who developed extrathoracic chronic GVHD [15]. Over a period of 3 years, we conducted a prospective cohort study that included a systematic follow-up of lung function for all of the consecutive patients who underwent an allogeneic HSCT in our center. Out of the 243 enrolled patients scheduled to be engrafted, 202 were included at Day 100, with an 18-month cumulative incidence of BO estimated at 12.9 % (Study NCT01219972 ALLOPULM clinical trials, analysis in progress; [17]).
Numerous risk factors for BO have been proposed in various retrospective studies, including age of the donor or recipient, type of transplant, degree of HLA incompatibility, presence of gastroesophageal reflux, gammaglobulin levels, type of GVHD prophylaxis, type of conditioning regimen, underlying blood disorder, tobacco use or acute GVHD, with conflicting results [8]. The only association reported in all of the studies was the occurrence of extrathoracic chronic GVHD at the time of BO diagnosis. One objective of our ALLOPULM study was to prospectively identify the events occurring within 3 months after transplantation that are associated with the subsequent occurrence of BO. Using multivariate analysis, we showed that a history of smoking, the occurrence of pulmonary infection, and pre-existing abnormal pre-transplant pulmonary function were associated with BO development [17].
Physiopathology of Bronchiolitis Obliterans
The exact pathogenesis of BO is still unknown. Epidemiological studies have shown an association between the development of BO and the presence of active chronic GVHD, which led to the hypothesis that BO is, in fact, chronic GVHD of the lung [18]. The most important mechanism contributing to BO would then result from the immune-mediated attack of airway epithelial cells by donor T cells. As opposed to acute GVHD, chronic GVHD also involves B-cell stimulation, autoantibody synthesis and systemic fibrosis [19]. Mouse models of chronic GVHD involve three disease mechanisms: autoantibody synthesis, pro-fibrotic processes and defective thymic function. Thymic damage leads to a decrease in TReg cell number and function and defective negative selection of T cells [19].
The recent development of an animal model of BO caused by allogeneic HSCT has revealed new pathophysiological pathways [20]: the peribronchiolar inflammatory infiltrate is mainly composed of CD4 lymphocytes, Clara cells that regenerate bronchiolar epithelium may be targets and a large number of cytokines are produced during the process [20]. More recently, the peribronchiolar deposition of alloantibodies was demonstrated in the same animal model, as was the role of mature B cells from the donor in the development of BO [21].
Some authors found that BO could be triggered by lower respiratory tract infections. In fact, it has been demonstrated that patients who present with respiratory syncytial or parainfluenza virus infection have an increased risk of developing BO in the year following HSCT [22]. The presence of a respiratory tract infection may lead to airway inflammation that causes an inappropriate alloimmune reaction.
Diagnosis of Bronchiolitis Obliterans
The definitive diagnosis of BO is pathologic. The National Institutes of Health Consensus proposed some histopathological criteria for BO based on a limited amount of original data [23]. The Pathology Working Group Report retained the presence of unequivocal dense eosinophilic scarring of the bronchioles resulting in some degree of luminal narrowing as a diagnostic (Fig. 32.1b). Inflammation is common but variable and insufficient for diagnosis [23]. In fact, Yousem et al. and Yokoi et al. published small pathological series in allogeneic HSCT recipients with chronic GVHD and lung involvement [24, 25] and reported different small airway abnormalities in this setting. Yousem et al. described two types of bronchiolar affections: lymphocytic bronchiolitis and cicatricial BO. Lymphocytic bronchiolitis is characterized by peribronchiolar/bronchiolar lymphocytic and plasmocellular infiltrate. Airway inflammation is predominantly lymphocytic and plasmocellular. Cicatricial BO is characterized by the obliteration of the airway lumen by dense fibrous tissue. The authors proposed that cicatricial BO is the late-stage of lymphocytic bronchiolitis [24]. The pathologic description of Yokoi et al. comes from eight autopsy cases. Small airway lesions varied from early inflammatory changes to late scarring in each case. The inflammatory lesions were usually mild and mostly lymphoplasmacytic, except in three patients with predominant neutrophilic infiltrates. In all cases, inflammatory and scarring stages were present simultaneously [25]. Although the gold standard for diagnosis of BO is the demonstration of bronchiolar lesions upon histological evaluation of a lung specimen, it is not current practice to obtain a lung biopsy. Transbronchial biopsies have poor sensitivity, and surgical lung biopsies are invasive and usually reserved for cases of confusing diagnosis. Thus, BO is usually diagnosed as a new fixed airflow obstruction demonstrated by pulmonary function testing (PFT). BO syndrome (BOS) is diagnosed based on clinical, functional and radiological evaluation of the patient.
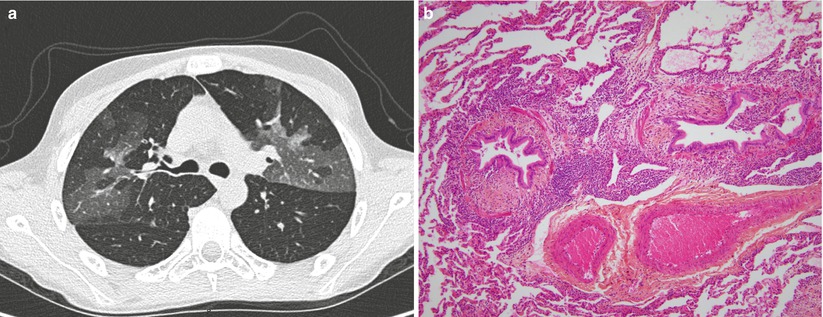

Fig. 32.1
Lung computed tomography (CT) scan (a) and lung biopsy (b) from a patient who was diagnosed with bronchiolitis obliterans 12 months after an allogeneic hematopoietic stem cell transplantation. The CT scan shows a mosaic pattern (a). The histological analysis shows a bronchiolar wall thickened by inflammatory fibrosis located between the epithelium and the smooth muscle. The airway lumen is narrowed (HES x 100) (b)
BOS clinically manifests as dyspnea at rest or on exertion, dry cough or wheezing. In a significant proportion of cases, it is asymptomatic and revealed by screening PFT. Clinical diagnoses of BOS are based on new-onset airflow obstruction identified by spirometry. Because BOS initial symptoms are nonspecific and spirometric findings are not sensitive, most patients are diagnosed when they have severe airway obstruction. PFT is not sensitive, as BOS is a distal airway disease, and bronchiolar obstruction needs to be widespread before FEV1 declines [26].
The currently used definition is that of the National Institute of Health (NIH) consensus guidelines for chronic GVHD, published in 2005: a 1-s forced expiratory volume (FEV1) value <75 % of that predicted and a FEV1/forced vital capacity (FVC) ratio <70 %, together with the exclusion of an infection and the presence of an extrathoracic sign of GVHD [5] (Table 32.3). However, some patients do not meet this functional criterion; despite the presence of BOS, the FEV1/FVC ratio may remain normal (>70 %). In fact, because BOS is a disease of the small airway, distal airway obstruction may lead to air trapping, thereby increasing the residual volume (RV). Consequently, the FVC declines concomitantly with the FEV1, and the FEV1/FVC ratio stays over 70 % [27]. NIH-BOS criteria include signs of air trapping, observed either by PFT (RV >120 %) or high-resolution computed tomodensitometry (HRCT) (Table 32.3). Recently, modification of these guidelines has been proposed [18]. To minimize the dynamic collapse of airways during the forced expiratory maneuver, Chien et al. proposed the use of slow vital capacity (SVC) instead of FVC for the diagnosis of airway obstruction. They also suggested that patients with annual FEV1 declines >5 % (from pre-transplant PFT) be considered at high risk of developing BOS and that an annual FEV1 decline >10 % (from pre-transplant PFT) be considered a diagnostic criterion for early BOS, even for a FEV1 >75 % of the predicted value [18]. Finally, two groups of patients may be diagnosed with BOS: one with a classical obstructive ventilatory defect and one with a normal FEV1/FVC ratio [18, 27]. The prognoses of both patients are similar [27]. Future studies should focus on the early detection of BOS. Chien et al. found that allogeneic HSCT recipients with annual FEV1 declines >5 %, even with FEV1/FVC ratios >80 %, have higher risks of nonrelapse mortality; this suggests that early airflow decline can be used as an indicator of early BOS [28]. This finding should prompt systematic serial PFT after transplant, with careful attention to patients who develop airflow decline over time.
Table 32.3
Consensus Criteria for diagnosis of bronchiolitis obliterans/bronchiolitis obliterans syndrome after allogeneic hematopoietic stem cell transplantation
2005 NIH Consensus Criteria [5] | Proposed modified Consensus Criteria [17] |
|---|---|
All the following should be met: | All the following should be met: |
1. FEV1 < 75 % of predicted normal and FEV1/FVC <70 %. | 1. FEV1 < 75 % of predicted normal. |
2. Either signs of air trapping by PFT (RV >120 % of predicted normal) or signs of air trapping, small airway thickening or bronchiectasis by in- and expiratory HRCT or pathological confirmation of constrictive bronchiolitis. | 2. FEV/VC <0.7. |
3. Absence of active respiratory tract infection. | 3. Evidence of air trapping on HRCT or RV >120 % of predicted normal. |
4. In case of lacking histological proof of BO, at least one other distinctive manifestation of cGVHD in an additional organ system is required. | 4. Absence of active respiratory tract infection. |
5. Presence of active cGVHD in another organ than the lung. | |
6. Decrease of the FEV1 by at least 10 % since pretransplant. | |
7. Use of slow vital capacity for calculation of the FEV1/VC ratio. |
In addition to PFT, HRCT should be performed. In the case of BOS, it may reveal a mosaic pattern suggestive of air trapping (Fig. 32.1a) that can be accentuated on expiratory cuts. It can also reveal bronchial thickening, bronchiectasis or bronchiolar nodules with a tree-in-bud pattern [5, 29]. Otherwise, HRCT is needed in the initial evaluation of symptomatic patients to eliminate other causes of respiratory symptoms, such as infectious or inflammatory pneumonitis. Its value in the follow-up of a patient diagnosed with BOS is limited unless new respiratory symptoms develop. Finally, a bronchoscopic exam should be performed to rule out infection in the presence of infiltrates at the HRCT. In the case of normal imaging studies, nasal aspirates, sputum stains and cultures are considered sufficient to rule out viral, bacterial or fungal disease [5].
BOS may also occur after lung transplantation as the result of a chronic graft rejection. Philit et al. have shown very similar clinical, imaging and functional features both in lung transplant and allogeneic HSCT recipients [30]. Thus, the studies in each of these situations contribute to a better understanding of both conditions.
Management of Bronchiolitis Obliterans Syndrome
BOS is a potentially fatal complication. Despite advances in the management of allogeneic HSCT recipients, the survival and treatment of patients with BOS have not improved over the last two decades. The overall survival rates at 2 and 5 years after allogeneic HSCT are respectively 45 and 15 % in patients who develop BOS [26]. The natural history of BOS is variable. Classically, PFT declines with time, and patients develop infectious complications, which can lead to respiratory insufficiency and, eventually, death. Some patients remain stable after the development of airway obstruction, and a minority (20 %) will respond to treatment [31]. Actual management is based on case series and expert opinion. Based on the assumption that BOS is a manifestation of chronic GVHD, current practice involves optimizing or reintroducing immunosuppressive therapy upon a diagnosis of BOS. Some reports suggest that high-dose systemic corticosteroids (1–2 mg/kg) can improve or at least stabilize the FEV1. If BOS develops upon tapering the immunosuppressive therapy, clinicians usually reintroduce the tapered drug in combination with corticosteroids. Some case reports/series suggest other immunosuppressive therapies, such as TNF-receptor blockade, imatinib and extracorporeal photopheresis (ECP), may be efficacious [26]. These therapeutic options need to be studied further. Converging data suggest the ineffectiveness of rituximab for BOS treatment [32, 33].
It is well known that long-term exposure to corticosteroids, even at low doses, leads to significant complications. To minimize the morbidity associated with this treatment, considering the low efficacy of steroids in this setting, some authors have suggested alternative agents with anti-inflammatory properties. The use of topical steroid treatment is supported by two retrospective studies. We reported decreases in dyspnea and improvements in FEV1 in seven patients with new-onset airflow obstruction treated with combined inhaled therapy (budesonide-formeterol) [34]. Bashoura et al. reported stabilization or improvement in FEV1 in 16/17 BOS patients treated 3–6 months with high-dose fluticasone [35]. A recent study suggests bronchodilator responsiveness in BOS patients [36]. This information may support the inhaled combination therapy, including a long-acting bronchodilator and a corticosteroid, over an inhaled corticosteroid alone. The efficacy of azithromycin in the treatment of BOS following allogeneic HSCT is controversial [37, 38]. Finally, the efficacy of montelukast as a corticosteroid-sparing agent in the treatment of chronic GVHD had been suggested in a pilot study [39]. These three agents are actually under study in BOS patients following HSCT, either alone or in combination [40].
Non Infectious Infiltrative Lung Diseases
Non infectious infiltrative lung diseases (ILDs) occurring late after allogeneic HSCT are not uncommon, representing 12 % to more than 60 % of late-onset non-infectious pulmonary complications in large retrospective studies, including OP [41, 42]. In the largest retrospective studies, ILDs after allogeneic HSCT are mostly described as OP, interstitial pneumonia or idiopathic pneumonia syndrome (IPS) [41, 43, 44]. Whereas OP has been well described in a large study [7], limited data are available on other forms of infiltrative lung diseases following allogeneic HSCT.
Idiopathic Pneumonia Syndrome
Idiopathic pneumonia syndrome (IPS) was first defined in 1993 by an NIH expert committee as diffuse alveolar opacities following allogeneic HSCT after lower respiratory tract infection or cardiac failure was excluded. This definition was recently updated to include new microbiological diagnostic tools for the exclusion of infection, especially for newly described pathogens (Table 32.4) [45]. Thus, this syndrome regroups different clinical entities that can be classified according to the primitively attempted lung compartment: parenchyma (acute interstitial pneumonitis (AIP), acute respiratory distress syndrome (ARDS), OP, iatrogenic lung injury) or pulmonary blood vessels (engraftment syndrome, capillary leak syndrome, diffuse alveolar hemorrhage). IPS is thought to result from a variety of lung insults, including the toxic effects of HSCT conditioning regimens, immunologic cell-mediated injury, inflammatory cytokines, and occult pulmonary infections [45]. Peri-engraftment respiratory distress syndrome (PERDS) occurring within 5 days of engraftment after allogeneic HSCT represents a clinical subset of IPS that should be identified because of its specific clinical characteristics and because its responsiveness to corticosteroids can lead to a good prognosis [45]. PERDS is the result of non-cardiogenic pulmonary edema with or without concurrent pleural effusions in the context of a diffuse capillary leak syndrome and dysfunction of other organs, such as the liver, kidney, skin or gut [45, 46].
Table 32.4
Diagnostic criteria of idiopathic pneumonia syndrome occurring after hematopoietic stem cell transplantation [42]
1. Evidence of widespread alveolar injury |
Multilobar radiologic infiltrates (chest X-ray, computed tomography) |
Symptoms and signs of pneumonia (cough, dyspnea, tachypnea, rales)
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|