Fig. 1.1
Myocardial infarction culminating in heart failure; the direct consequences of myocardial infarction and the subsequent local and peripheral responses designed to protect the body from the effects of the failing heart (Adapted from McKay RG, Pfeffer MA, Pasternak RC, Markis JE, Come PC, Nakao S, et al. Left ventricular remodeling after myocardial infarction: a corollary to infarct expansion. Circulation. 1986;74(4):693–702)
Direct injury to the myocardium with subsequent loss of contractile function can also be seen with infiltrative processes such as toxins, infections, and genetic abnormalities (these will be discussed further in the section on “Heart Failure with Normal/Preserved Ejection Fraction”).
(b)
Hemodynamic Stress on the Ventricular Myocardium
Left ventricular systolic dysfunction also develops secondarily to the hemodynamic stress of a chronic pressure load (termed “afterload”) or volume load (termed “preload”) on the ventricular wall. Increased afterload is observed in patients with aortic stenosis and in patients with uncontrolled hypertension. Similarly, increased preload is seen in patients with chronic mitral and/or aortic insufficiency, intracardiac shunts, and arteriovenous fistulas. In response to these disease processes, which impose sustained hemodynamic stress on the ventricular wall, the heart muscle undergoes a pathological hypertrophy (Fig. 1.2). This is the early phase of remodeling. In cases of increased afterload, the ventricle undergoes concentric hypertrophy, which is characterized by an increased ventricular wall thickness in comparison to wall cavity size. In cases of increased preload, the ventricle undergoes eccentric hypertrophy, characterized by an increase in chamber volume with normal or reduced wall thickness [5].
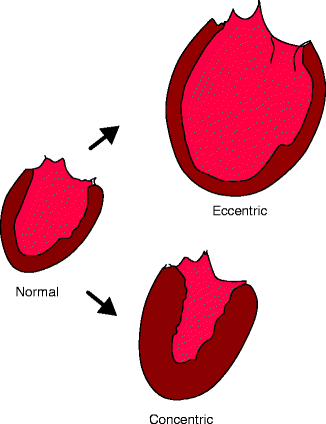

Fig. 1.2
Maladaptive cardiac hypertrophy: concentric and eccentric hypertrophy compared to a normal heart (Adapted from Katz AM. Physiology of the Heart. 3rd ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2001])
Whereas cardiac hypertrophy can be a normal physiologic response to exercise, allowing for an increase in mass and improvement in contractility, pathologic hypertrophy involves no improvement in contractility. Rather, it allows the ventricle to maintain contractile force temporarily until it can no longer overcome the increased hemodynamic stress. As mentioned, the increased wall stress also promotes the production of inflammatory cytokines. These cytokines have been shown to have deleterious effects on contractile proteins by altering their expression and by triggering pathways involved in myocyte apoptosis. Cytokine and neurohormonal production have been shown to occur in later phases of remodeling. Eventually the muscle fibers accumulate collagen and fibrose [6]. This eventually leads to left ventricular dilatation, further loss of contractile function, and thus reduced systolic function (Fig. 1.3).
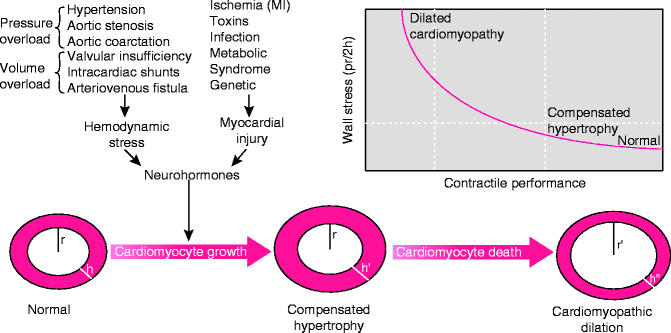

Fig. 1.3
The response of the heart muscle to stress. Hypertrophy of the cardiac muscle preserves contractile function initially, but eventually, the hypertrophied muscle fibroses and gives way to dilatation and loss of contractile function (Adapted from Diwan A, Dorn GW 2nd. Decompensation of cardiac hypertrophy: cellular mechanisms and novel therapeutic targets. Physiology (Bethesda). 2007;22(1):56–64)
Heart Failure with Normal/Preserved Ejection Fraction
Diastolic dysfunction and diastolic HF have different meanings. In both cases, the ventricle becomes less compliant, leading to impaired/abnormal ventricular filling, as measured by echocardiography or other imaging modalities. Diastolic dysfunction refers only to impaired/abnormal filling by imaging; diastolic HF instead refers to diastolic dysfunction with clinical symptoms and signs of HF. To more clearly make a distinction between these two entities, the term “diastolic HF” is now substituted by a relatively new construct referred to as Heart Failure with Normal/Preserved Ejection Fraction (HFNEF). Approximately 50 % of the overall HF population has a normal left ventricular ejection fraction (LVEF). In comparison to patients with HF and low LVEF, these individuals are more likely to be women and more likely to be older. They also have a higher likelihood of obesity, hypertension, renal failure, atrial fibrillation, and anemia [7]. The clinical syndrome of HF in these individuals can be as profound as those patients with HF symptoms and low LVEF [8]. Similarly, the prognosis of patients with clinical HF and normal LVEF is only minimally better in comparison to those with patients with a low LVEF [9].
These two entities also share common etiologies. As mentioned, aortic stenosis and poorly controlled hypertension often lead secondarily to left-sided heart failure. Prior to the development of left-sided HF, the ventricle remodels via a mechanism of concentric hypertrophy, known as left ventricular hypertrophy (LVH), as it works to preserve cardiac output. With LVH, there is often impaired ventricular relaxation and thus higher ventricular filling pressures. LVH is therefore a common cause of HFNEF since higher ventricular filling pressures can cause “backup” of fluid into the lungs despite normal LV contractility. The other major causes of HFNEF are also attributable to impaired ventricular relaxation and include transient myocardial ischemia, infiltrative processes that deposit into the myocardial architecture creating a restrictive cardiomyopathy, and hypertrophic cardiomyopathy [10]. Infiltrative processes involve the intercalation of toxins, diseases, or infections into the myocardium. The following are examples of common infiltrative sources: chemotherapy, amyloidosis and other connective tissue diseases, alcohol from long-term abuse, and human immunodeficiency virus (HIV) and other viruses. Genetic and myopathic disorders such as Duchenne Muscular Dystrophy can also produce a restrictive cardiomyopathy [11].
As mentioned, patients with left-sided HF and HFNEF not only share similar etiologies but often have similar clinical presentations. However, the mechanisms by which the left ventricle acts to maintain stroke volume in these two groups of patients are different. In HF with low LVEF, the eccentric or dilated left ventricle acts to maintain stroke volume via the Frank-Starling mechanism (Fig. 1.4). By this mechanism, the left ventricle’s increased compliance accommodates for greater ventricular filling and thus a greater end-diastolic volume (EDV). This permits a greater stroke volume with each subsequent contraction and thus a way to preserve forward cardiac output, although only to a certain degree. Comparatively, in HFNEF, the left ventricle is in a remodeling phase and is able to maintain contractile function and normal stroke volume but must do so at elevated ventricular filling pressures. As a result of the elevated pressures, the EDV will be normal or reduced (Fig. 1.4).
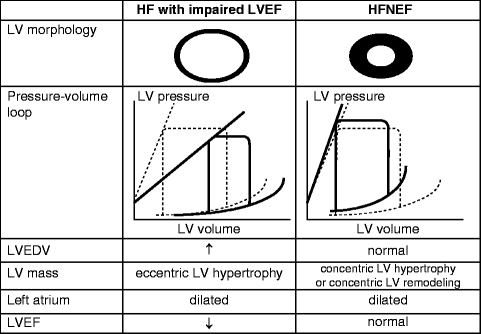

Fig. 1.4
Pressure and volume changes throughout different stages of ventricular remodeling in heart failure (Adapted from Maeder MT, Kaye DM. Heart failure with normal left ventricular ejection fraction. J Am Coll Cardiol. 2009;53(11):905–918)
Although their adaptive mechanisms are different, HFNEF actually exists in a continuum with left ventricular systolic dysfunction. A good example of this continuum is the ventricle’s response to afterload. As described in earlier sections, in response to an afterload like aortic stenosis, the heart will undergo remodeling likely via concentric hypertrophy or LVH. During this period, the patient will often present with HFNEF, prior to the loss of contractile myocytes and left-sided HF. Conversely, patients with left-sided HF may also present with a significant component of diastolic dysfunction, owing to impaired ventricular filling from a greater EDV [12].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


