Pneumonia in the Immunocompetent Host
Despite advances in medicine, pneumonia remains a major cause of morbidity and mortality. It affects six million American adults each year. With a mortality rate of 13.4 per 100,000, it is the sixth most common cause of death in the United States (1). Community-acquired pneumonia (CAP) in previously healthy individuals is caused by bacteria in 25% of patients, atypical pathogens (including mycoplasma) in 25%, and viruses in 20%. A few percent more feature multiple causative organisms, but many patients are treated without conclusive evidence.
In hospitalized adults with pneumonia, 60% to 80% are due to Streptococcus pneumoniae, up to 20% are caused by gram-negative bacteria, a few percent result from Staphylococcus aureus, and the remainder are due to viruses and Mycoplasma. For nosocomial or hospital-acquired pneumonia, gram-negative organisms are responsible for 35% to 40%, S. pneumoniae for 10%, S. aureus for 3%, and no agent is identified in approximately 40%.
The diagnosis of pneumonia is based on a combination of clinical history and examination, serologic tests, and diagnostic imaging (2,3,4,5,6) (Boxes 5.1 and 5.2). It is usually not possible to identify the etiology based on clinical or radiologic findings alone. Serologic tests require a few days. It is therefore important that the clinician and radiologist work together, pooling information and resources to narrow the differential diagnosis. The radiologist’s role is to detect abnormality, define its location and extent, evaluate for complications,
and monitor response to therapy. As in most clinical settings, the radiologist is significantly aided by having relevant clinical information.
and monitor response to therapy. As in most clinical settings, the radiologist is significantly aided by having relevant clinical information.
Box 5.1: Common Features of Bacterial Pneumonias
Respiratory symptoms predominate
Acute onset of symptoms
Focal signs on clinical examination
Correlation between clinical and radiologic findings
Pathologic changes in a focal area or areas of lung parenchyma
Box 5.2: Features of Atypical and Viral Pneumonia
Systemic symptoms predominate over respiratory symptoms
No response to usual antibiotic therapy
Discrepancy between the severity and location of clinical signs and radiologic findings
Pathologic changes mainly in the bronchial tree and interstitial parenchyma
Clinical Clues to the Cause of Pneumonia
Accurate early treatment is important in many of the bacterial pneumonias if complications (and even death) are to be avoided. The choice of an appropriate antibiotic rests on the radiologic and clinical findings. Several factors help to narrow the diagnostic possibilities. Patient age is one such factor. Viruses are the usual cause of pneumonia in children. Mycoplasma occurs mainly in children and young adults. Bacteria most commonly cause adult pneumonias.
Clinical symptoms can also be useful. An acute history of productive cough, pleuritic chest pain, and chills is characteristic of bacterial pneumonia (7). On physical examination, focal signs are often present, and there is a high incidence of radiologic findings. In atypical pneumonias systemic symptoms such as fatigue, malaise, arthralgias, and low-grade fever predominate, with a more gradual onset (8). There is often a discrepancy between the clinical and radiologic findings.
Predisposing factors may point to specific organisms. Alcoholism, dementia, neuromuscular disease, swallowing disorders, and general anesthesia predispose to aspiration pneumonia. Anaerobic organisms, gram-negative bacteria, and S. aureus are most often isolated. Chronic obstructive pulmonary disease (COPD) exacerbations are often caused by Hemophilus influenzae or Branhamella catarrhalis. Pneumonia complicating influenza is often caused by S. aureus. S. pneumoniae is common in sickle cell disease and in patients who have previously undergone splenectomy. Pseudomonas aeruginosa and S. aureus are commonly responsible agents in cystic fibrosis. Immunocompromised patients are considered separately in Chapter 6.
Imaging Modalities
Chest radiographs (CXR) are the first line of defense and the mainstay for imaging suspected pneumonia. If pneumonia is confirmed clinically and radiologically, therapy is generally begun. In elderly patients and heavy cigarette smokers, follow-up CXR is advised to complete resolution of disease, thereby excluding postobstructive pneumonia. Radiologic resolution may lag behind clinical improvement by as much as 6 to 8 weeks (and even longer in elderly and COPD patients). Ultrasound can be used to evaluate pleural opacity seen on CXR. The largest areas of pleural fluid can be marked radiologically for aspiration.
Radiologic resolution of bacterial pneumonia often takes 6 to 8 weeks.
Chest computed tomography (CT) can be helpful in patients with pneumonia that resolves too slowly or fails to resolve. A small mass may be obscured by obstructive atelectasis on CXR. CT is better at detecting small calcifications and enlarged intrathoracic nodes. CT with contrast enhancement helps to differentiate lung abscess from empyema when CXR is equivocal.
Radiologic Signs of Pneumonia
Opacification
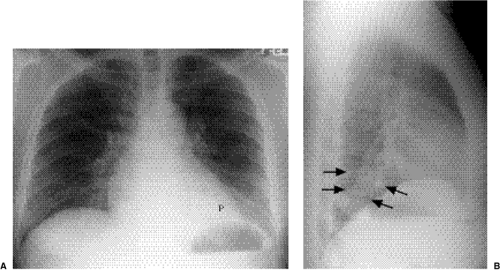
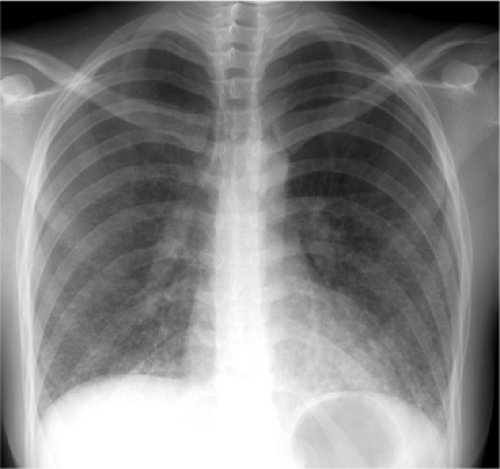
Focal opacity may be visible, especially when comparing one lung with the other on the frontal projection. On the lateral projection attention should be directed over the thoracic spine, the cardiac silhouette, and the retrosternal and retrocardiac regions, where faint opacity may otherwise escape detection (Figs. 5.1 and 5.2).
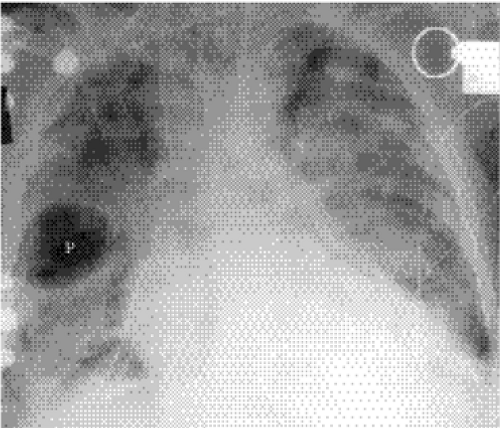
 Figure 5.1 Opacity in pneumonia. (A) Posteroanterior and (B) lateral chest radiographs: minimal right basilar opacity, much better seen on the lateral view overlying the heart (P). |
Silhouette Sign
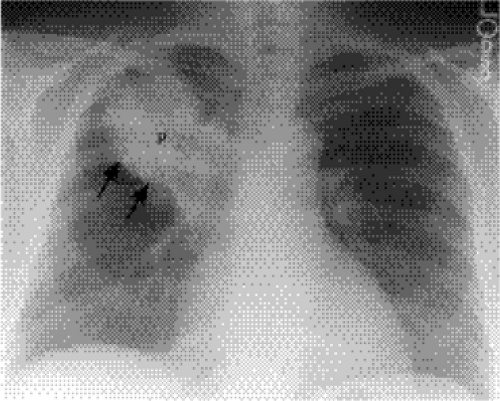
Lung opacity contacting a mediastinal or diaphragmatic border will obliterate or silhouette that border (“silhouette sign”). Right middle lobe opacity can be subtle on the frontal CXR, necessitating careful scrutiny of the right heart border (Fig. 5.3). Lingular disease obliterates the left heart border (Fig. 5.4), whereas lower lobe opacity may obscure a hemidiaphragm.
Airspace Diseaes
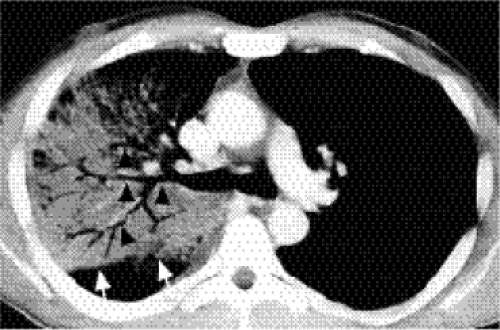
This describes replacement of the airspaces with fluid or exudate without gross destruction or displacement of lung morphology. Signs of airspace disease (Chapter 14) (Fig. 5.5)
occur. At CT early airspace disease may manifest as ground glass opacity, with increased attenuation but with pulmonary vessels still visible.
occur. At CT early airspace disease may manifest as ground glass opacity, with increased attenuation but with pulmonary vessels still visible.
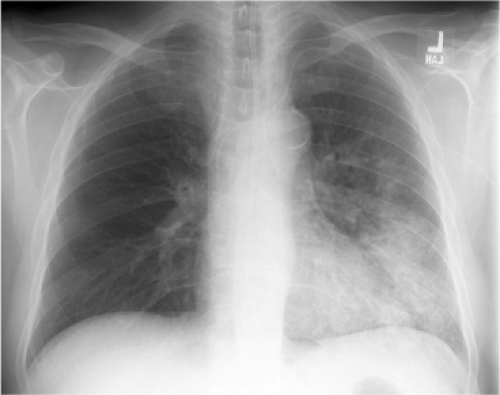 Figure 5.4 Silhouette sign of lingular pneumonia. Partial loss of visualization of lower left heart border. |
Peribronchial Thickening
Perceptible thickening of a bronchus is most commonly seen in viral infections. It can often be discerned by comparing the bronchus diameter with its accompanying pulmonary artery.
Atelectasis
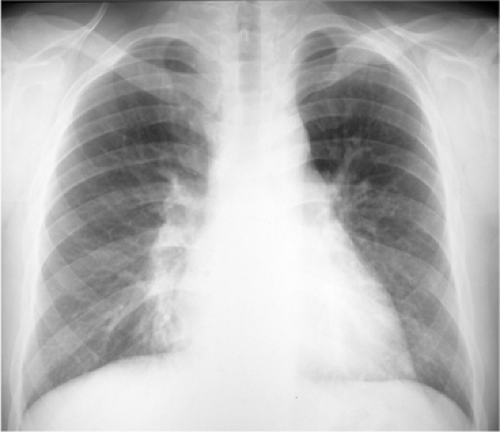
This describes loss of lung volume (Fig. 5.6), and the term collapse is used when a whole lobe or lung is involved. Mild atelectasis occurs in many patients, especially those with interstitial pneumonia. It usually manifests as linear or discoid atelectasis.
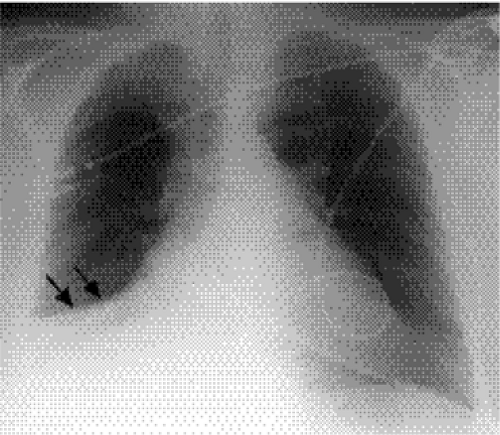 Figure 5.6 Atelectasis with pneumonia. Depression of major fissure (arrows) and silhouetting of right heart border indicate combined right middle and lower lobe atelectasis. |
Radiologic Classification of Pneumonia
Pneumonia is recognized to have different radiologic appearances. These generally have no bearing on the final diagnosis, because the same organism may produce several patterns. In addition, the early use of antibiotics alters the evolution of these patterns. The patterns are as follows:
Bronchopneumonia: This is the most common pattern. Here, the inflammatory exudates are mainly related to the central bronchovascular structures and may be multifocal (Fig. 5.7). Some acini may be spared, resulting in a patchy distribution of opacity. There is often associated volume loss.
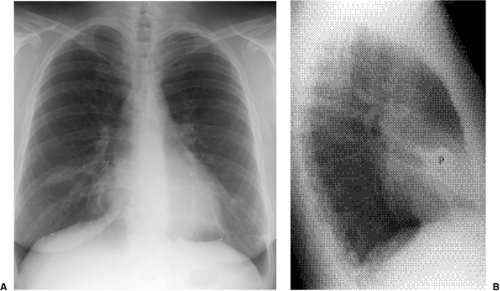
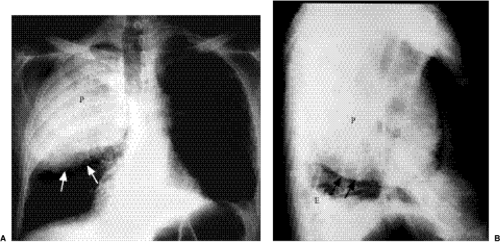
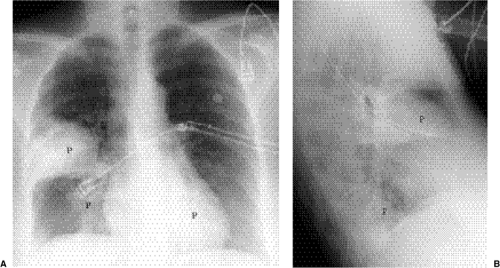
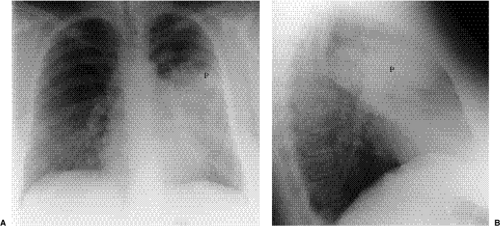 Figure 5.8 Lobar pneumonia. (A) Posteroanterior and (B) lateral chest radiographs demonstrate airspace opacity filling much of the left upper lobe (P). |
Lobar pneumonia: The exudate begins distally and spreads circumferentially, giving rise to more homogeneous opacity (Figs. 5.8 and 5.9). It may eventually involve the whole lobe. The airways are not principally involved, so there is little volume loss, and air bronchograms are generally present.
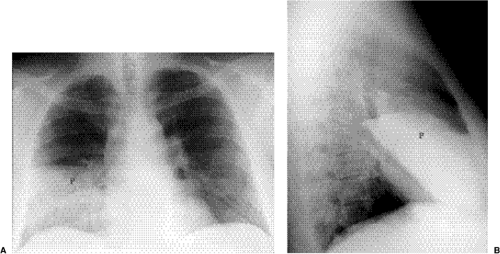 Figure 5.9 Lobar pneumonia. (A) Posteroanterior and (B) lateral chest radiographs demonstrate opacification of virtually the entire middle lobe (P). |
Interstitial pneumonia: This consists of peribronchial thickening and ill-defined reticulonodular opacities (Fig. 5.10). This pattern is typical of Mycoplasma and viruses. Patchy distribution of atelectasis is often seen.
Round pneumonia: Some pneumonias have this configuration initially. There is usually an ill-defined border (Fig. 5.11). Air bronchograms are frequently present.
Differential Diagnosis of Pneumonia
Acute airspace disease has a reasonably brief differential diagnosis. Pneumonia generally differs from pulmonary edema in its more focal distribution. There is generally not associated cardiac enlargement in pneumonia. At CT pneumonia demonstrates nondependent opacity (as opposed to the dependent pattern of pulmonary edema). Pulmonary edema also improves or worsens in hours as opposed to days in the case of pneumonia.
Adult respiratory distress syndrome (ARDS) is often indistinguishable from pneumonia on a single radiographic study. Over time changes will occur more slowly in ARDS, and it is often more widespread than pneumonia. CT will show dependent changes in ARDS.
Subsegmental atelectasis may be indistinguishable from early pneumonia. The more linear the radiographic abnormality, the less likely it represents pneumonia. Associated clinical signs and symptoms may be helpful; transmitted breath sounds are often amplified in pneumonia but not in atelectasis.
The radiographic appearance of pulmonary infarct may be quite similar to pneumonia. Enlargement of a pulmonary artery or attenuated peripheral vessels may suggest a vascular etiology. Infarct is more likely to be well marginated at its borders and typically evolves more rapidly than pneumonia.
The appearance of pulmonary hemorrhage is very similar to pneumonia. There is often a history of frank hemoptysis. Clearance of opacity usually occurs more quickly than in pneumonia.
Some neoplasms, such as lymphoma and bronchoalveolar carcinoma, produce airspace opacification indistinguishable from pneumonia. Serial studies show more chronic abnormality with gradual change. Lymph node enlargement at CXR would be more typical of lymphoma than pneumonia.
Radiologic Clues to the Cause of Pneumonia
Lobar consolidation is often due to bacteria. Postobstructive pneumonia needs to be considered in patients at risk for lung carcinoma. Expansive consolidation occurs in Klebsiella pneumonia (“Friedlander pneumonia”) (Fig. 5.12) but can also be seen with S. pneumoniae.
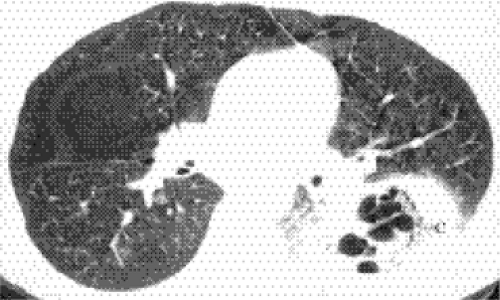
Cavitating consolidation (Figs. 5.13 and 5.14) suggests bacteria or fungi. S. aureus, Klebsiella, anaerobes, and Mycobacterium tuberculosis commonly cause cavitation. Pneumatoceles may result in a similar appearance (Fig. 5.15) and suggest S. aureus or S. pneumoniae. Emphysematous bullae within consolidated lung may mimic cavities.
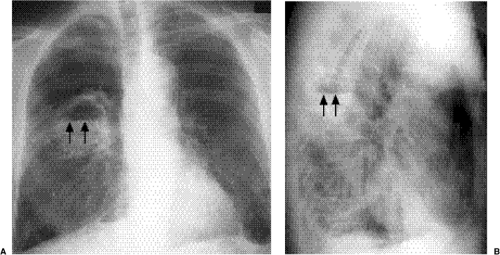 Figure 5.13 Cavitary pneumonia. (A) Posteroanterior, and (B) Lateral chest radiographs: cavity with a gas-fluid level (arrows) in the superior segment of the right lower lobe. |
Nodular or spherical pneumonia is usually due to pneumococcal, Legionella, Q fever, or fungal pneumonia. Hematogenous dissemination of S. aureus can cause spherical pneumonia or septic emboli. Reticulonodular opacity and peribronchial thickening are typical of viruses and Mycoplasma.
Patchy consolidation with a dependent distribution occurs with aspiration pneumonia. This is usually bilateral. Patchy upper lobe disease is suggestive of tuberculosis (TB) or histoplasmosis, especially if there is associated cavitation. Miliary opacities occur with overwhelming tuberculous or fungal infection.
Dependent pneumonia suggests aspiration, whereas cavitary upper lobe disease raises the possibility of TB.
Pleural effusions may develop with anaerobic bacteria, gram-negative bacteria, S. aureus, and S. pyogenes. Empyema should be suspected when pleural effusion is large or loculated or develops late in the course of disease.
Complications
Pleural effusions/empyema: these are indistinguishable radiographically.
Cavitation: if enlarging or greater than 4 cm in diameter, an indication for intervention.
Pneumatoceles: usually self-limiting and resolve within 4 weeks. They resemble bullae and blebs.
Pneumothorax: seen in Pneumocystis carinii pneumonia (Chapter 6).
Lymph node enlargement: seen with fungal and mycobacterial infections. In patients at risk, also consider bronchogenic carcinoma.
Bone destruction: may occur in actinomycosis, nocardiosis, fungal infections, and TB.
Abscess: may be difficult to distinguish from empyema. CT sometimes helpful to differentiate these two entities
Bronchiectasis: any chronic or severe infection may cause bronchiectasis, and in cystic fibrosis patients, upper lung bronchiectasis tends to occur.
Expansive consolidation:
Klebsiella
Haemophilus influenzae
Pneumococcal pneumonia
Plague pneumonia
TB
Lung abscess: S. aureus, Klebsiella
Lung mass
Cavitating pneumonia:
S. aureus
Klebsiella
TB
Aspiration
Actinomycosis
Nocardiosis
Histoplasmosis
Aspergillosis
Coccidioidomycosis
Echinococcosis
Amoebiasis
Pneumatoceles:
S. aureus
S. pneumoniae
Escherichia coli
Klebsiella
H. influenzae
P. carinii
Legionella pneumophila
Bacterial Pneumonia
Streptococcus pneumoniae (Pneumococcus)
This gram-positive coccus is responsible for anywhere from 10% to 80% of cases of CAP (9,10). It is the most frequent organism resulting in hospitalization for pneumonia. It is common in healthy young adults, typically presenting with chills, fever, and cough productive of rust-colored blood-tinged sputum. Spread is by the airborne route. Diagnosis can be made by sputum or blood culture, although pneumococci are found in the sputum of 10% to 40% of normal patients. Sputum cultures are negative in nearly half of patients with positive blood cultures. Most patients are diagnosed presumptively, based on clinical and radiographic presentation, without the need for cultures.
Pneumococcus is the organism most frequently resulting in hospitalization for pneumonia.
Homogeneous airspace disease confined to a single lobe with an irregular margin (Fig. 5.16) is the most common radiographic pattern, seen in about one-third of patients. Pneumococcal pneumonia is one of the causes of expansive consolidation with bowing of fissures. More widespread patchy bronchopneumonia is seen in another one-third, usually confined to a single lobe. Interstitial opacification simulating viral or atypical pneumonia occurs in one-fourth of patients. The remainder show a mixed patchy and interstitial pattern. There is a predilection for the lower lobes. Pleural effusions are seen in one-third. Consolidation in pneumococcal pneumonia is said to clear by central “lysis.” Patients with shorter clinical histories tend to show more rapid radiographic resolution. As in all pneumonias there is a lag period between clinical resolution and radiologic resolution, which can be several weeks.
Staphylococcus aureus
This gram-positive coccus is an uncommon but serious cause of CAP (11,12,13). It has a fulminant course with fever, cough, dyspnea, purulent sputum, hemoptysis, and chest pain. Spread occurs by the airborne or hematogenous route. When acquired via inhalation, S. aureus pneumonia is often a complication of influenza during epidemics. Airborne spread
is also implicated in debilitated patients. Hematogenous spread occurs secondary to soft tissue infections, valve prostheses, hemodialysis, and intravenous drug use. Morbidity is high, with a reported mortality rate of 7% to 19%.
is also implicated in debilitated patients. Hematogenous spread occurs secondary to soft tissue infections, valve prostheses, hemodialysis, and intravenous drug use. Morbidity is high, with a reported mortality rate of 7% to 19%.
Initially, radiographs most commonly (75%) show multilobar homogeneous airspace disease (Fig. 5.17). Subsequent radiographic deterioration is often seen. Bilateral changes are noted in 35%. Cavitation or abscess formation (25%), pneumatoceles (40%), pleural
effusions (33%), and pneumothorax (20%, often associated with pleural effusion or empyema) are other common findings. Pneumonia with pneumatocele or pneumothorax should suggest S. aureus as the cause.
effusions (33%), and pneumothorax (20%, often associated with pleural effusion or empyema) are other common findings. Pneumonia with pneumatocele or pneumothorax should suggest S. aureus as the cause.
Bacillus anthracis
This is a gram-positive sporulating nonmotile organism. It is endemic in the soil of Texas, Oklahoma, and the lower Mississippi valley (14). Cutaneous disease accounts for over 90% of cases, with gastrointestinal and inhalational disease causing 5% each. Inhalational disease results from industrial or agricultural exposure to animal hides, hair, wool, or bone meal of contaminated livestock. The disease is most prevalent among herbivores such as cattle, sheep, horses, and goats. Prior radiation exposure, alcoholism, and underlying pulmonary disease are thought to be risk factors. Spores of 2 to 5 μm reach the alveoli. Macrophages engulf the spores and transfer them to mediastinal and hilar nodes. Germination occurs with production of large amounts of anthrax toxin. This spills over into the systemic circulation with resultant edema, hemorrhage, necrosis, and septic shock. Massive hemorrhagic mediastinitis occurs. Inhalational anthrax manifests as an initial flu-like illness followed by rapidly progressive respiratory failure and death.
CXRs show widening of the mediastinum due to hemorrhagic mediastinitis and lymphadenitis (15,16). Pleural effusions also occur. Focal hemorrhagic pneumonia is only seen in one-fourth of patients. CT better demonstrates hemorrhagic mediastinitis and necrosis. It also excludes other etiologies of widened mediastinum.
Klebsiella
This gram-negative bacillus is also known as Friedlander bacillus (17). It is found in the upper respiratory tract of 2% to 25% of healthy persons. It is an uncommon cause of pneumonia and a well-known cause of biliary and urinary tract sepsis. Middle-aged men
are most commonly affected, and many patients have predisposing conditions such as alcoholism, malnutrition, and diabetes. Chronic pulmonary diseases such as asthma and bronchiectasis may also predispose to Klebsiella infections. The incubation period is short. Cough, pleuritic pain, and fever are frequent initial symptoms, with malaise, chills, and shortness of breath also occurring. The mortality rate is high, estimated at 70% to 80%. Of the remainder, a few recover slowly, whereas others suffer chronic disease with a clinical course similar to TB.
are most commonly affected, and many patients have predisposing conditions such as alcoholism, malnutrition, and diabetes. Chronic pulmonary diseases such as asthma and bronchiectasis may also predispose to Klebsiella infections. The incubation period is short. Cough, pleuritic pain, and fever are frequent initial symptoms, with malaise, chills, and shortness of breath also occurring. The mortality rate is high, estimated at 70% to 80%. Of the remainder, a few recover slowly, whereas others suffer chronic disease with a clinical course similar to TB.
Radiographs most commonly show scattered lobular foci of airspace disease that may coalesce to form larger opacities (18,19). Typically, opacity has a sharp advancing border. This pattern is indistinguishable from other bacterial pneumonias. The upper lobes are involved in two-thirds of cases. Healing generally leaves residual scarring and distortion of lung. Radiographs may show expansion of a lobe (Fig. 5.12) due to “drowned lung.” Shift of the mediastinum to the opposite side may occur with whole lung involvement. Small pleural effusions are thought to occur because pleural thickening often remains after resolution of acute changes. Empyema occurs in about 6%. These patients have the worst clinical course. Klebsiella deserves consideration whenever opaque, extensive, expansive airspace disease is seen.
Klebsiella should be considered with opaque, extensive, expansive airspace disease.
Pseudomonas aeruginosa
This gram-negative organism is a rare cause of CAP in healthy persons (20,21). Pseudomonas colonizes bronchiectatic airways, especially in cystic fibrosis. It is sometimes present on normal skin and is also a secondary contaminant in wounds. Prematurity, chemotherapy, antibiotics, steroids, immunosuppressants, old age, and debility increase the risk of Pseudomonas. Neutropenia increases the risk of severe disease. Most cases occur in hospitalized patients, especially those on ventilators. Organisms have been cultured from sinks, catheters, receptacles, ventilator equipment, and staff. Pseudomonas is the most common pathogen isolated from the lower respiratory tract of ventilated patients and is implicated in 25% of ventilator-associated pneumonias. Mortality in these patients is in
the 80% to 100% range. Pneumonia is usually secondary to aspiration of oropharyngeal contents in intensive care unit patients. Sedation, endotracheal intubation, and intermittent positive pressure ventilation predispose to infection. Person-to-person contact has been implicated in hospitals.
the 80% to 100% range. Pneumonia is usually secondary to aspiration of oropharyngeal contents in intensive care unit patients. Sedation, endotracheal intubation, and intermittent positive pressure ventilation predispose to infection. Person-to-person contact has been implicated in hospitals.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree