Perioperative Evaluation and Management of Patients with Known or Suspected Cardiovascular Disease Who Undergo Noncardiac Surgery: Introduction
Each year in the United States, approximately 27 million patients undergo noncardiac surgery.1 Of these, approximately 50,000 experience perioperative myocardial infarction (MI), and more than half of the 40,000 perioperative deaths are caused by cardiac events.2,3 As the population of the United States continues to age over the next several decades, both the total number and the percentage of patients who are older than 65 years of age will increase. These patients represent the largest group in whom surgeries are performed, a group in which approximately 25% of surgeries are associated with significant risk of cardiac morbidity and death and a group at increased risk for the presence of cardiac disease. As such, the number of patients with significant perioperative risk undergoing noncardiac surgery can be expected to increase.
Most perioperative cardiac morbidity and deaths are related to myocardial ischemia, congestive heart failure, or arrhythmias. Therefore, preoperative evaluation and perioperative management to reduce morbidity and mortality rates emphasize the detection, characterization, and treatment of coronary artery disease (CAD), left ventricular (LV) systolic dysfunction, and significant arrhythmias. However, not all patients with underlying cardiac disease are at significantly increased perioperative risk of a morbid cardiac event. The purpose of preoperative evaluation is not to clear patients for surgery but to assess their medical status, cardiac risks posed by the surgery planned, and recommend strategies to reduce risk. Evaluation must be tailored to the circumstances that have prompted the consultation and to the nature of the surgical illness. There are two goals of the preoperative evaluation: (1) to identify patients at increased risk of an adverse perioperative cardiac event and (2) to identify patients with a poor long-term prognosis because of cardiovascular disease who come to medical attention only because of the problem requiring noncardiac surgery. In this sense, the preoperative evaluation represents an opportunity to identify and treat patients, thereby affecting long-term prognosis, even though their risk at the time of noncardiac surgery may not be prohibitive.
Preoperative evaluation can identify many patients at increased risk of an adverse cardiac event, and appropriate perioperative management can reduce that risk. Internists and cardiologists play vital roles in the evaluation and management of patients before, during, and after noncardiac surgery. This chapter reviews available data and recommendations for the preoperative evaluation and perioperative management of patients with known or suspected cardiovascular disease undergoing noncardiac surgery. The nature of preoperative evaluation and perioperative management should be individualized to the patient and the clinical scenario surrounding surgery. Patients presenting with an acute surgical emergency require only a rapid preoperative assessment, with subsequent management directed at preventing or minimizing cardiac morbidity and death. Among such patients, a more thorough evaluation can often be performed after surgery. In contrast, patients undergoing elective procedures with no surgical urgency can undergo a more thorough preoperative evaluation. Among patients presenting for cardiac evaluation before “same-day” elective surgery, perioperative risk to the patient must be weighed against the impact of additional testing and cancellation or delay of the surgical procedure.
Clinical Determinants of Perioperative Cardiovascular Risk
The majority of patients at increased risk of adverse perioperative cardiac events can be identified using a simple bedside or office assessment. A careful history, physical examination, and review of the resting 12-lead electrocardiogram (ECG) are usually sufficient to allow stratification of most patients into low, intermediate, or high risk for an adverse perioperative cardiac event. A number of investigators have established readily accessible clinical markers that predict increased perioperative risk of MI, congestive heart failure, or death.4-17 Some investigators have used a quantitative scoring system to rank the importance of individual risk factors.5,6,9 The advantage of such systems rests with the observation that some clinical features are stronger predictors of perioperative risk than are others. Recommendations of the American College of Cardiology (ACC) and American Heart Association (AHA)18 designate risks factors as belonging to three groups: major, intermediate, and minor (Table 87–1). In the guidelines, greater weight is given to active than to quiescent disease, and the severity of disease is used to modify its importance.
| Major predictors |
| Acute or recent MIa with evidence of ischemia based on symptoms or noninvasive testing |
| Unstable or severeb angina (Canadian class III or IV) |
| Decompensated heart failure |
| High-grade AV block |
| Symptomatic ventricular arrhythmias with underlying heart disease |
| Supraventricular arrhythmias with uncontrolled ventricular rate |
| Severe valvular heart disease |
| Intermediate predictors |
| Mild angina pectoris (class 1 or 2) |
| Prior MI by history or Q waves on ECG |
| Compensated or prior heart failure |
| Diabetes mellitus (particularly insulin dependent) |
| Renal insufficiency (creatinine ≥2.0 mg/dL) |
| Minor predictors |
| Advanced age |
| Abnormal ECG (LV hypertrophy, left bundle-branch block, ST-T abnormalities) |
| Rhythm other than sinus (eg, atrial fibrillation) |
| Low functional capacity (inability to climb one flight of stairs with a bag of groceries) |
| History of stroke |
| Uncontrolled systemic hypertension |
Historical features are important in the identification of patients at increased perioperative cardiac risk. Because most perioperative morbidity and deaths are related to myocardial ischemia, congestive heart failure, and arrhythmias, the assessment of historical risk factors relies heavily on the recognition of CAD, LV dysfunction, and significant arrhythmias. Risk factors recognized as predictive of increased perioperative risk18 include advanced age; poor functional capacity; and history of CAD, congestive heart failure, arrhythmia, valvular heart disease, diabetes mellitus, uncontrolled systemic hypertension, renal insufficiency, and stroke. CAD is a major risk factor in the setting of recent MI or unstable or severe angina pectoris and an intermediate risk factor in the setting of mild stable angina pectoris or remote MI. Similarly, congestive heart failure is a major risk factor if decompensated and an intermediate risk factor if compensated. A history of arrhythmias may be a major, intermediate, or minor risk factor, depending on the nature and severity of the arrhythmia as well as the presence of underlying heart disease.
A patient’s preoperative functional capacity significantly influences the assessment of perioperative cardiac risk. Good functional capacity in an asymptomatic patient predicts low perioperative risk despite the presence of other risk factors. Impaired functional capacity is important in three regards in the assessment of perioperative cardiac risk. First, among patients with chronic CAD and among those who have experienced an acute cardiac event, poor functional capacity is associated with an increased risk of subsequent cardiac morbidity and death.16 Second, many of the historical features that predict increased perioperative risk assume physical activity. Because most symptoms of cardiac disease are either associated exclusively with or exacerbated by increased physical activity, significant noncardiac limitations in physical capacity are associated with inherent problems in the ability to detect symptoms of underlying cardiac diseases and thereby to diagnose them. Finally, poor functional capacity is associated with impaired conditioning and therefore a lesser ability to accommodate the cardiovascular stresses that may accompany noncardiac surgery. Because the ability to perform tasks in daily activities correlates well with maximal oxygen uptake on treadmill testing, the assessment of functional capacity on preoperative history is an important feature in the assessment of perioperative risk.
Features on physical examination may be useful in assessing perioperative risk. Patients with uncontrolled systemic hypertension should be identified and treated. Because congestive heart failure9,18,19 and valvular heart disease9,18,19 are associated with increased risk, physical findings suggestive of these diagnoses should be sought. The physical examination should include the patient’s general appearance (cyanosis, pallor, dyspnea during conversation or minimal activity, Cheyne-Stokes respiration, poor nutritional status, obesity, skeletal deformities, tremor, and anxiety), blood pressure in both arms, carotid pulses, extremity pulses, and ankle-brachial indices. Jugular venous pressure and positive hepatojugular reflex are reliable signs of hypervolemia in chronic heart failure; pulmonary rales and chest radiographic evidence of pulmonary congestion correlate better with acute heart failure. Patients with aortic stenosis can be identified by a typical murmur with diminished and delayed upstroke of the carotid or brachial pulse. Patients with mitral stenosis, mitral regurgitation, or aortic regurgitation may be at increased perioperative risk of developing congestive heart failure in the setting of sufficiently severe disease and are at increased risk of infective endocarditis. Finally, the presence of carotid or other vascular bruits helps identify patients at increased risk of occult CAD.
A patient’s overall health affects his or her perioperative cardiovascular risk; associated medical conditions may exacerbate risk or complicate perioperative cardiac management. Patients with diabetes mellitus have an increased risk of concomitant CAD, and the possibility of silent ischemia complicates both the preoperative recognition of CAD and the perioperative recognition of ischemia. Patients with either restrictive or obstructive pulmonary disease are at increased risk of perioperative respiratory complications, and the associated hypoxemia, hypercapnia, acidosis, and increased work of breathing can exacerbate cardiac stress and precipitate myocardial ischemia. Patients with preexisting renal dysfunction may be predisposed to volume retention in the perioperative period, and hypovolemia may lead to renal hypoperfusion, thereby exacerbating renal dysfunction. Patients with anemia of any cause are at increased risk of myocardial ischemia and congestive heart failure mediated by increased cardiac stress and increased cardiac work. Optimal management of noncardiac conditions may therefore reduce the risk of cardiac morbidity in the perioperative period.
Perioperative cardiac risk is related in two ways to the type of noncardiac surgery being performed. First, some types of noncardiac surgery identify a group of patients at increased risk for concomitant cardiac disease based on shared risk factors that predispose patients to both noncardiac and cardiac disease. The most notable example of this relationship is seen with vascular surgery and CAD. In this case, the same factors that result in clinical peripheral arterial occlusive disease also predispose to the development of CAD. Among such patients, CAD may be known or occult, with no symptoms because of the physical limitations associated with significant peripheral vascular disease. Second, the nature of noncardiac surgery may be associated with variable degrees of cardiac stress, mediated by fluctuations in heart rate, blood pressure, intravascular volume, and oxygenation as well as the cardiac stresses associated with the duration of the procedure, pain, and neurohumoral activation.4,6,7,9,20-23 Emergency procedures are associated with a two- to five-fold increase in perioperative cardiac risk compared with elective procedures.3,19 Other types of noncardiac surgery associated with high perioperative risk include aortic and peripheral vascular surgery and prolonged abdominal, thoracic, or head and neck procedures with large fluid shifts. The ACC/AHA Task Force Report on Perioperative Cardiac Evaluation18 stratifies noncardiac surgical procedures as involving high, intermediate, and low cardiac risk (Table 87–2).
| High risk (reported cardiac riska >5%) |
| Emergency major operations, particularly in elderly patients |
| Aortic, major vascular, and peripheral vascular surgery |
| Extensive operations with large volume shifts or blood loss |
| Intermediate risk (reported cardiac risk <5%) |
| Intraperitoneal and intrathoracic |
| Carotid endarterectomy |
| Head and neck surgery |
| Orthopedic |
| Prostate |
| Low riskb (reported cardiac risk <1%) |
| Endoscopic procedures |
| Superficial biopsy |
| Cataract |
| Breast surgery |
The perioperative administration of anesthesia may also affect perioperative cardiac risk. Although there is no one best myocardial protective anesthetic technique,24-28 differences in anesthetic techniques may favor the use of one over another for individual patients. Opioid-based general anesthesia generally does not affect cardiovascular function, although the commonly used inhalational agents cause afterload reduction and decreased myocardial contractility. Spinal anesthesia results in sympathetic blockade, with decreases in both preload and afterload and the potential for shifts in both systemic blood pressure and intravascular volume. In general, hemodynamic effects are minimal when spinal anesthesia is used for infrainguinal procedures, but higher dermatomal levels of spinal anesthesia, as required for abdominal procedures, may be associated with significant hemodynamic effects, including hypotension and reflex tachycardia. No study has clearly demonstrated any beneficial change in outcome from the use of pulmonary artery catheters, ST-segment monitoring, or transesophageal echocardiography. Decisions regarding specific anesthetic technique and intraoperative monitoring are best left to the anesthesiologists involved in the patient’s care.
Preoperative Testing
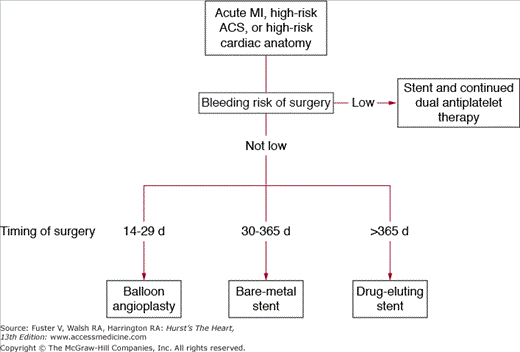
Patients at very low risk and those at high risk of an adverse perioperative cardiac event can typically be identified using clinically available features described above. Patients at low risk generally require no additional testing before noncardiac surgery. Among patients undergoing elective noncardiac surgery in whom risk is determined to be intermediate or high, additional testing may be useful to better define their risk.18 It is well to use a stepwise approach to the preoperative assessment of cardiac risk (Fig. 87–1). Testing usually includes noninvasive testing and very rarely coronary angiography to assess for the presence and significance of CAD, LV function, and valvular heart disease.
Figure 87–1.
Proposed treatment for patients requiring percutaneous coronary intervention who need subsequent surgery. ACS, acute coronary syndromes; MI, myocardial infarction. Reproduced with permission from Fleisher et al.114
Impaired LV systolic or diastolic function is predictive of perioperative congestive heart failure. The greatest risk of complications occurs among patients with LV ejection fraction of less than 35%; among critically ill patients, severely impaired LV systolic function is associated with a higher risk of death. Preoperative LV systolic function can be assessed noninvasively using radionuclide ventriculography or echocardiography, or it may be assessed invasively using contrast ventriculography. Unless recently defined, preoperative assessment of LV systolic function should be performed among patients with poorly controlled congestive heart failure and should be considered among patients with prior congestive heart failure and among patients with dyspnea of unknown cause.
Preoperative cardiac stress testing is useful in the objective assessment of functional capacity to help identify patients at risk of perioperative myocardial ischemia or cardiac arrhythmias and to aid in the assessment of long-term as well as perioperative prognosis. In general, poor functional capacity may be attributable to advanced age, deconditioning, myocardial ischemia or other causes of reduced cardiac reserve, or poor pulmonary reserve. Reduced functional capacity identifies patients at increased risk of subsequent cardiac morbidity and death.29 The clinical history can be effectively used to estimate functional capacity. In addition, preoperative exercise testing is a useful tool to objectively assess functional capacity as well as to assess hemodynamic response to stress and the potential for stress-induced myocardial ischemia or cardiac arrhythmias.
In a general population, the mean sensitivity and specificity of exercise ECG studies for the detection of CAD are 68% and 77%, respectively, with reported ranges of sensitivity from 23% to 100% and specificity from 17% to 100%.30 The accuracy of exercise ECG studies for the detection of CAD is influenced by the prevalence of disease in the population studied; the degree of exercise achieved; and the number, location, and severity of diseased vessels. The mean sensitivity and specificity for the detection of multivessel disease are 81% and 66%, respectively.31
In addition to assessment for the presence of CAD, exercise testing is useful for the assessment of prognosis. In a large cohort of 4083 medically treated patients in the Coronary Artery Surgery Study (CASS),32 exercise testing was useful for identifying both high- and low-risk subgroups of patients. The mortality rate was 5% per year or more among a high-risk subset comprising 12% of the total population who were unable to achieve an exercise workload greater than Bruce stage I and had an abnormal exercise ECG. In contrast, mortality was less than 1% per year among a low-risk subset comprising 34% of the total population who were able to achieve at least Bruce stage III with a normal exercise ECG. Preoperative exercise testing has been shown to be useful in the prediction of perioperative cardiac risk among patients undergoing peripheral vascular surgery, abdominal aortic aneurysm repair, and other major noncardiac surgery.33-43 In these published reports, the negative predictive value for perioperative death or MI was 91% to 100%, with a positive predictive value of 0% to 81%.
Many patients undergoing noncardiac surgery are unable to exercise. Approximately 30% to 50% of patients undergoing noncardiac surgery are unable to achieve an adequate exercise workload for a diagnostic study. This is especially problematic among patients with peripheral vascular occlusive disease, in whom the same factors that cause peripheral disease predispose them to coronary atherosclerosis; significant peripheral vascular disease severely limits exercise tolerance and therefore the ability to perform diagnostic exercise stress testing. For this reason, pharmacologic stress testing may offer advantages in the preoperative testing of some patients undergoing peripheral vascular surgery as well as of other patients who are not able to perform adequate physical exercise because of noncardiac limitations.
Pharmacologic stress testing for the detection of CAD can be performed using one of several methods. Infusion of the adrenergic agonist dobutamine leads to increases in heart rate; myocardial contractility; and to a lesser degree, blood pressure, resulting in increased myocardial oxygen demand. In the setting of a limited oxygen supply, increased demand causes myocardial ischemia. Dobutamine infusion is typically used in conjunction with echocardiographic imaging, and the failure of wall motion to augment with dobutamine or for it to become frankly dyskinetic indicates of ischemia. Alternatively, pharmacologic “stress” can be achieved using the coronary vasodilators dipyridamole or adenosine. Nuclear perfusion imaging, such as thallium scintigraphic imaging, is typically used in conjunction with dipyridamole and adenosine. CAD is detected as heterogeneity of perfusion in response to maximal coronary vasodilation. Stress imaging using rapid multislice computed tomography or magnetic resonance imaging is likely to be used more often in the coming years.
Dipyridamole thallium scintigraphy has been extensively studied for the assessment of CAD and perioperative risk among patients undergoing vascular,7,35,44-58 and other noncardiac surgery.47,59-64 Published reports found a uniformly high negative predictive value for perioperative morbidity associated with normal dipyridamole thallium scintigraphic results, with values ranging from 95% to 100% and an average value of approximately 99%. The positive predictive value of dipyridamole thallium redistribution for MI or death from cardiac causes has been reported to be from 4% to 20% among studies including more than 100 patients. There is also important long-term prognostic value associated with preoperative nuclear perfusion imaging,49,58,65 suggesting that late postoperative risk after uncomplicated noncardiac surgery can also be predicted by preoperative testing. Although any abnormality on dipyridamole thallium scintigraphy is suggestive of CAD and is associated with a higher perioperative cardiac risk compared with patients with normal scan results, perioperative cardiac risk associated with a fixed perfusion defect is substantially lower than that associated with perfusion redistribution. In addition, the size of a perfusion defect is directly related to the patient’s perioperative cardiac risk.46,49,51
Dobutamine stress echocardiography is well established for the noninvasive detection and characterization of CAD,66-71 with an overall predictive accuracy equivalent to that of dipyridamole thallium scintigraphy. Several studies have evaluated the utility of dobutamine stress echocardiography for preoperative assessment of patients undergoing vascular or other noncardiac surgery.72-77 Negative predictive values for perioperative events ranged from 93% to 100%. Positive predictive values were 17% to 43% for any cardiac event and 7% to 23% for predicting MI or death. As was seen with studies using nuclear perfusion imaging, most studies of dobutamine stress echocardiography did not blind treating physicians to stress test results, and subsequent alteration of patient management based on abnormal noninvasive test results presumably contributed to a low event rate despite a positive test result. A meta-analysis of preoperative pharmacologic stress tests78 demonstrated similar power of dobutamine stress echocardiography and dipyridamole thallium scintigraphy in predicting adverse cardiac events after noncardiac surgery.
Because clinical factors are usually able to identify patients at low or high risk for an adverse cardiac event after noncardiac surgery,18,20 preoperative stress testing typically has the greatest utility among patients at intermediate risk. Exercise ECG study allows assessment of functional capacity as well as evaluation for evidence of CAD based on ST-segment analysis and hemodynamics. Performance of exercise echocardiographic testing or exercise nuclear perfusion imaging should be considered in the presence of significant resting ECG abnormalities that preclude diagnostic testing for CAD, such as left bundle-branch block, LV hypertrophy with strain, or digitalis effect. Nonexercise stress testing, such as dobutamine stress echocardiographic or dipyridamole thallium scintigraphic studies, should be considered among patients who are unable to perform adequate physical exercise.
The performance of preoperative noninvasive testing should be based on an assessment of risk and benefit to the patient. In this setting, benefit is defined as the likelihood that testing may alter management and improve outcome because of an adverse perioperative or long-term prognosis. Risk to the patient should include risk associated with additional procedures precipitated by noninvasive testing as well as any risk associated with the noninvasive testing. With the high costs associated with many evaluation strategies, development and implementation of evidence-based guidelines may lead to more efficient and cost-effective use of appropriate noninvasive testing.
As noted above, clinical features can be used to identify patients at very low risk for an adverse perioperative cardiac event, including asymptomatic patients having undergone coronary revascularization within 5 years as well as those without specific clinical markers for increased risk. Additional testing of selected patients at intermediate or higher risk can potentially reduce the cost of testing without affecting patients’ outcomes. Based on a previous study validating the use of selective noninvasive testing before major aortic surgery,79 the cost implications of selective testing were assessed in the ACC/AHA Task Force report.18 In the previous study, the application of a clinical algorithm resulted in only 29% of 201 patients undergoing noninvasive testing before aortic surgery, with an associated 0.5% perioperative cardiac mortality rate. Using estimated costs, the use of selected testing was associated with a total cost of $32,886 for 58 patients compared with an estimated total cost of $113,967 if all 201 had undergone noninvasive screening. Froehlich et al80 and Almanaseer et al81 demonstrated that implementation of the ACC/AHA cardiac risk assessment guidelines appropriately reduced resource use and costs in patients who underwent elective aortic surgery without affecting outcomes.80,81 Preoperative stress testing (88%-47%; P <.00001), cardiac catheterization (24%-11%; P <.05), and coronary revascularization (25%-2%; P <.00001) were all significantly reduced with appropriate use of the ACC/AHA guidelines.80,81 The low perioperative mortality rate associated with the use of a clinical algorithm and selected noninvasive testing suggest that substantial cost can be avoided without compromising patients’ safety.
Preoperative Therapy for Coronary Artery Disease
CAD is responsible for the majority of adverse perioperative cardiac events. When disease is recognized, specific therapy should be instituted to minimize the risk of perioperative myocardial ischemia, MI, or death.
There is limited information regarding the impact of either preoperative coronary artery bypass grafting or percutaneous coronary intervention (PCI) on perioperative cardiac morbidity and mortality rates. Several retrospective studies suggest that patients with successful prior coronary revascularization have a low risk of perioperative cardiac events during noncardiac surgery and that the risk of death is comparable to that among patients with no clinical evidence of CAD.82-89
Although these studies support the theory that coronary artery revascularization lowers the risk of adverse cardiac events associated with noncardiac surgery, they do not address the overall effect on morbidity and mortality rates associated with the surgical coronary revascularization. In the assessment of patients undergoing noncardiac surgery, the well-established long-term benefits of coronary artery bypass surgery or PCI should be considered, as should any impact on noncardiac surgical morbidity and mortality rates. There may be an occasional patient for whom coronary artery bypass grafting should be performed before noncardiac surgery only because of an otherwise prohibitive perioperative cardiac risk. However, there are many more patients with advanced CAD who are candidates for surgical coronary revascularization, based on long-term prognosis, for example, with critical left main trunk stenosis who are identified only during preoperative cardiac assessment. Among such patients, elective noncardiac surgery of intermediate or high risk should generally be postponed for the performance of coronary artery bypass surgery.
Several small, retrospective studies82,85,86 have suggested that there is a low risk of perioperative MI or death after preoperative PCI. One study of 1049 noncardiac surgeries performed among 1829 patients enrolled in the Bypass Angioplasty Revascularization Investigation (BARI) trial demonstrated a low incidence of MI or death among patients having undergone either coronary artery bypass surgery or PCI, with an event rate of 1.6% among patients in both groups.90 The absence of any evident difference between groups suggests that previous PCI confers protection from perioperative cardiac events that is similar to that conferred by surgical revascularization, assuming that patients have been followed closely and that recurrent ischemia has been effectively treated.
The Coronary Artery Revascularization Prophylaxis (CARP) randomized trial demonstrated that coronary artery revascularization using either bypass surgery or PCI before elective vascular surgery did not alter long-term survival.91 Although the study was not powered to detect a beneficial effect in the short term, there was no reduction in the number of postoperative MIs, deaths, or days in the hospital. The results mirror those of other randomized clinical trials in the nonoperative setting that have shown that electivecoronary revascularization in “low-risk” patients who have stable CAD does not provide a survival benefit and does not reduce the risk of late MI as compared with excellent medical and preventive therapies.
The Dutch Echocardiographic Cardiac Risk Evaluation Applying Stress Echo (DECREASE)-V pilot study randomized 101 high-risk patients by the presence of three or more clinical risk factors and extensive ischemia on dobutamine stress echocardiography to best medical therapy and revascularization or best medical therapy alone before vascular surgery.92 The outcome of 30-day all-cause death or nonfatal MI was similar between the revascularization and medical therapy patients at 43% versus 33% (odds ratio [OR], 1.4; 95% confidence interval [CI], 0.7-2.8; P = .30), respectively. On the basis of these data, coronary artery revas cularization before elective vascular surgery among patients with stable cardiac symptoms cannot be routinely recommended.
Overall, indications for coronary revascularization among patients undergoing preoperative evaluation should be considered the same as for the general population.18 These include patients who have poorly controlled angina pectoris despite maximal medical therapy and patients with one of several high-risk coronary characteristics, that is, clinically significant stenosis (>50%) of the left main coronary artery, severe two- or three-vessel CAD (>70% stenosis) with involvement of the proximal left anterior descending coronary artery, easily induced myocardial ischemia on preoperative stress testing, or LV systolic dysfunction at rest. Current evidence suggests that PCI before noncardiac surgery is of no value in preventing perioperative cardiac events except in patients in whom PCI is independently indicated for an acute coronary syndrome. Unscheduled noncardiac surgery in a patient who has undergone a prior PCI presents special issues, particularly with regard to management of the dual-antiplatelet agents required in those who have received coronary stents.
Coronary stents are now used in more than 80% of coronary interventions, presenting unique challenges because of the risk of coronary thrombosis and bleeding during the initial recovery phase. In a cohort of 40 patients who received stents within 30 days of noncardiac surgery, all eight deaths and seven MIs, as well as eight of 11 bleeding episodes, occurred in patients who had undergone surgery within 14 days after stent placement.93 The complications appeared to be related to serious bleeding resulting from postprocedural antiplatelet therapy or from coronary thrombosis in those who did not receive 4 full weeks of dual antiplatelet therapy after stenting. Wilson et al94 demonstrated that 4.0% of patients undergoing surgery 6 weeks after stent placement died or had a MI or stent thrombosis with no events in the patients undergoing surgery 7 to 9 weeks after stent placement. These data suggest that, whenever possible, noncardiac surgery should be delayed 6 weeks after bare-metal stent placement, by which time stents are generally endothelialized, and a course of antiplatelet therapy to prevent stent thrombosis has been completed.94 Poststenting therapy currently includes a combination of aspirin and clopidogrel for at least 4 weeks followed by aspirin for an indefinite period. Drug-eluting stents (DES) should not be implanted before planned noncardiac surgery unless surgery can be safely performed on dual antiplatelet therapy or elective noncardiac surgery can be delayed for 12 months to allow effective post-DES antiplatelet therapy. If thienopyridines must be discontinued before major surgery, aspirin should be continued and the thienopyridine restarted as soon as possible. Figure 87–1 suggests proposed treatment algorithm for patients requiring PCI who need subsequent surgery.