Percutaneous Coronary Intervention: Introduction
The treatment of patients with coronary heart disease changed dramatically with the development of surgical coronary artery revascularization techniques in the 1970s and with percutaneous coronary intervention (PCI) in the next decade, performed initially with balloon angioplasty and then, beginning in 1986, with metallic stents and then, in 2003 in the United States, with drug-eluting stents (DESs). This chapter addresses the development and contemporary use of catheter-based coronary artery intervention, including selection of patients and devices, procedural issues, adjunctive therapy, results, complications, and future directions.
Development of Balloon Angioplasty
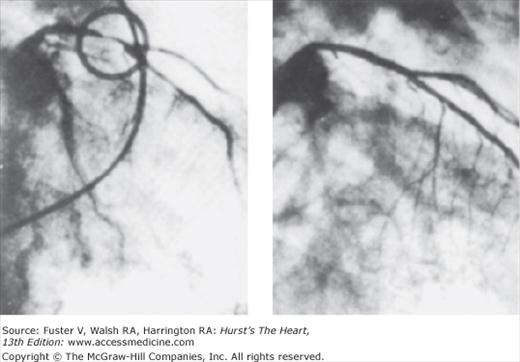
Percutaneous transluminal coronary angioplasty (PTCA) was conceived and shepherded into worldwide acceptance by Andreas R. Gruentzig, but the stage was set by the pioneering work of Dotter and Judkins,1 who in 1964, mechanically dilated femoral arteries with a coaxial double-catheter system, and of Zeitler et al,2 who applied this technique successfully in West Germany and introduced it to Gruentzig. After Gruentzig’s development of a polyvinyl chloride balloon catheter with fixed maximal inflated diameters in 1974, balloon angioplasty evolved rapidly.3-5 In September 1977, the first PTCA was performed in Zurich in a 37-year-old insurance salesman with severe angina pectoris and high-grade stenosis of the proximal left anterior descending (LAD) coronary artery.6-8 Balloon angioplasty was successful in relieving the stenosis, and on the 10th and 23rd anniversaries of this landmark procedure, coronary arteriography revealed wide patency of the LAD, and stress testing remained normal (Fig. 62–1).9
Figure 62–1.
Right anterior oblique coronary arteriogram of the first patient who underwent transluminal coronary angioplasty on September 16, 1977 (left) and on September 16, 1987 (right). During this 10-year period, the patient remained completely asymptomatic, and the arteriogram at 10 years showed no narrowing in the coronary arteries. Subsequent angiographic follow-up at 23 years revealed continued patency of this first percutaneous transluminal coronary angioplasty site.9
Following the report of Gruentzig’s first five patients in 197810 and 50 patients in 1979,11 worldwide interest in the technique was assured. Under the auspices of the National Heart, Lung, and Blood Institute (NHLBI), multicenter registries were formed to report experiences with the evolving technique of coronary angioplasty.12,13 Development of an over-the-wire balloon catheter by Simpson et al,14 combined with advances in guidewire and balloon catheter technology, resulted in steerable balloon catheter systems capable of crossing and dilating heretofore unreachable coronary stenoses. The use of percutaneous revascularization (PCI) exceeded 130,000 procedures in the United States in 1986, 400,000 in 1995, 1,000,000 in 1999, and 1,313,000 in 2006, approximately a three-fold dominance over the use of surgical revascularization.
Randomized Trials of Balloon Angioplasty
The favorable results of observational studies reporting outcomes in single-vessel and multivessel diseased patients15,16 led to a series of randomized trials comparing balloon angioplasty with medical therapy and with coronary artery bypass graft (CABG). The Angioplasty Compared to Medical Therapy Evaluation (ACME) and second Randomized Intervention Treatment of Angina (RITA-2) trials randomized mostly stable, single-vessel disease patients reporting better angina relief with angioplasty with similar rates of death or myocardial infarction (MI).17,18 Three trials, Fast Revascularization During Instability in Coronary Disease (FRISC II), Treat Angina With Aggrastat and Determine Cost of Therapy With Invasive or Conservative Strategy (TACTICS-TIMI-18), and RITA-3, supported an invasive approach that has become the treatment of choice for this patient subset (see Non–ST-Segment Elevation Acute Coronary Syndrome section).19-21
In mildly symptomatic patients in the Atorvastatin Versus Revascularization Treatment (AVERT) trial, PTCA was compared with aggressive lipid-lowering therapy (atorvastatin 80 mg).22 At the 18-month follow-up, angina relief was significantly better in the PTCA group, but quality-of-life scores were similar. In AVERT, stents were used in 30% of patients. This study suggests that in low-risk patients with no or mild symptoms, aggressive lipid lowering is as effective as PTCA with limited stenting in reducing subsequent ischemic events and emphasizes the importance of extending aggressive lipid lowering in all patients with obstructive coronary artery disease (CAD). The Medicine, Angioplasty, or Surgery Study (MASS-I) randomized 214 patients with stable angina, normal left ventricular function, and severe proximal LAD stenosis to bypass surgery, PTCA (without stent use), or medical therapy.23 At 3 years, there was no difference in death or MI. Both revascularization strategies yielded better symptom relief, but subsequent procedures were more common in the PTCA group. In MASS-II, 611 patients with stable angina, multivessel disease, and preserved left ventricular function were randomized to medical therapy, CABG, or PTCA.24 Symptom relief was better with PTCA and CABG than with medical therapy at 1 year. There was no difference in survival at 5 years, but there was less subsequent revascularization in the surgical group (3.5%) than in the medical group (24.2%) or PCI group (32.2%).25
More than 5000 patients were randomized in trials comparing coronary angioplasty with CABG surgery (Table 62–1). Two of these trials were sponsored by the NHLBI and performed in the United States. The first, the Emory Angioplasty Versus Surgery Trial (EAST), was a single-center study,26 whereas the larger Bypass Angioplasty Revascularization Investigation (BARI)27 involved 18 centers. In-hospital mortality was similar for angioplasty and bypass surgery (~1%) in these two studies of patients with multivessel disease, and 5-year survival also was similar. Repeat revascularization procedures, however, were more common in the angioplasty group, occurring in >50% of patients compared to 8% to 16% of CABG patients. Meta-analyses of eight randomized published trials comparing PTCA and CABG (BARI not included) reported no difference in mortality or MI at 1 year after angioplasty or CABG, but 18% of the angioplasty patients had required bypass surgery, and 20% had an additional angioplasty, a significantly higher rate of repeat revascularization than in the surgery group.28,29 This increased need for additional revascularization procedures in angioplasty patients, largely as a consequence of restenosis, eroded the initial cost advantage of angioplasty; by 3 years in the EAST study, angioplasty had been 95% as costly as bypass surgery,30 and at 8 years, total costs were $46,548 for CABG and $44,491 for PTCA.31 Importantly, however, the ability to undergo a low-risk initial CABG was preserved for the vast majority of PTCA patients.
| Balloon angioplasty vs CABG |
| EAST (Emory Angioplasty Versus Surgery Trial) |
| BARI (Bypass Angioplasty Revascularization Investigation) |
| CABRI (Coronary Angioplasty Versus Bypass Revascularization Investigation) |
| RITA (Randomized Intervention Treatment of Angina) |
| GABI (German Angioplasty Bypass Surgery Investigation) |
| ERACI (Argentine Randomized Study of Coronary Angioplasty) |
| PCI with stenting vs CABG |
| ARTS (Arterial Revascularization Therapies Study) |
| SOS (Stent or Surgery) |
| ERACI II |
| MASS-II (Medicine Angioplasty or Surgery Study)a |
| AWESOME (Angina With Extremely Serious Operative Mortality Evaluation)b |
| PCI with drug-eluting stenting vs CABG |
| ARTS II |
| SYNTAX (Synergy Between Percutaneous Coronary Intervention With Taxus and Cardiac Surgery) |
| CARDia (Coronary Artery Revascularization in Diabetics)c |
| FREEDOM (Future Revascularization Evaluation of Patients With Diabetes Mellitus: Optimal Management of Multivessel Disease) |
Considerable interest was generated by a subset analysis of treated diabetics in BARI. Among the 353 diabetics treated with insulin or oral hypoglycemic agents, 5-year survival was significantly better in patients who underwent surgery compared with patients who underwent PTCA.32 Analysis of 7-year survival for all patients in BARI revealed for the first time a significantly better survival with CABG compared with PTCA. Although there was no difference in the survival of nondiabetics after PTCA and CABG, there was a poorer survival of treated diabetics revascularized with PTCA.33 Further analysis of treated diabetics in BARI revealed that the survival benefit with CABG was conferred only to patients who received an internal mammary artery graft. At 8 years, EAST, which initially showed no difference between PTCA and CABG in diabetics, showed the same trend as BARI.34 Development of new lesions, perhaps unrecognized, probably accounted for those events occurring many years after revascularization.
Metallic Coronary Stents
Development of stainless steel intracoronary stents and, subsequently, DESs, profoundly influenced interventional cardiology. The first coronary stents were implanted in 1986 by Puel in Toulouse and Sigwart in Lausanne for restenosis prevention,35,36 an unproven hypothesis at the time, whereas the initial implantation in a patient in the United States was performed by the investigators at Emory University in 1987 in the setting of abrupt closure,37,38 after encouraging results in a canine model by Roubin et al.39 The initial European experience was with a self-expanding mesh stent, whereas the experience at Emory was with a balloon-mounted coil stent that was approved by the US Food and Drug Administration (FDA) for abrupt or threatened closure in 1993. This stent made balloon angioplasty considerably safer by providing effective therapy for coronary dissections and reducing the need for emergency coronary bypass surgery, but the use of this stent, despite intensive anticoagulation with heparin and warfarin, was complicated by stent thrombosis in 5% to 10% of patients, and bleeding was a common complication.
The device that revolutionized interventional cardiology in the 1990s was the Palmaz-Schatz stent (Johnson & Johnson Interventional Systems, Warren, NJ). On the basis of two carefully conducted randomized trials that showed reduced restenosis compared with balloon angioplasty,40,41 this device was granted FDA approval for marketing in 1994 for the elective treatment of de novo lesions in native coronary arteries. More than 100,000 implantations of this stent were performed in the first year of its availability. The interest in stenting was greatly heightened by a pivotal observation by Colombo et al42 that complete stent expansion by high-pressure balloon inflation, confirmed by intravascular ultrasound, and with substitution of the antiplatelet agents aspirin and ticlopidine for warfarin, yielded a very low stent thrombosis rate and fewer hemorrhagic complications. Subsequently, a series of observational and randomized trials confirmed the superiority of thienopyridines and aspirin and ultimately showed that clopidogrel was safer than ticlopidine and equally effective.
A number of randomized trials comparing stents and PTCA were conducted using dual antiplatelet therapy. The Belgium Netherlands Stent II (BENESTENT II) study that randomized patients to the heparin-coated Palmaz-Schatz stent or standard balloon angioplasty found better event-free survival at 12 months, lower restenosis, and $1020 in higher costs in stent patients at 1 year.43 The Optimal Angioplasty Versus Primary Stenting (OPUS-1) trial randomized 479 patients to primary stenting or balloon angioplasty followed by provisional stenting when necessary and reported that after 6 months, the combined incidence of death, MI, and target-vessel revascularization was significantly lower in the primary stenting arm and, at 6 months, primary stenting was slightly less expensive.44 This provocative study favored routine stenting when the anatomy was appropriate. Routine stent implantation was also supported by a meta-analysis of 29 published, randomized trials of routine stenting versus balloon angioplasty. This analysis, which involved 9918 patients, showed no difference in death, MI, or the need for coronary bypass surgery, but the need for repeat PCI was reduced by routine stenting (odds ratio [OR], 0.59).45 Routine stenting resulted in the avoidance of a repeat procedure in approximately 5 patients per 100 treated. The use of coronary stents was reviewed extensively in American College of Cardiology (ACC)/American Heart Association (AHA) guideline statements and in other reports.46-49
In a meta-analysis of 11 randomized trials comparing PCI with medical treatment in patients with stable CAD, Katritsis and Ioannidis50 reported no evidence that PCI with bare-metal stents prevented death, cardiac death, non-fatal MI, or need for subsequent revascularization. The aim of the Clinical Outcomes Utilizing Revascularization and Aggressive Drug Evaluation (COURAGE) Trial was to assess the effectiveness of PCI with bare-metal stents when added to optimal medical therapy compared with optimal medical therapy alone in patients with stable coronary disease.51 A total of 2287 patients were randomly assigned to PCI plus medical therapy or medical therapy alone. Before randomization, all patients had coronary angiography, and 86% had stress testing; patients with very positive stress tests, left ventricular dysfunction, and severe CAD were excluded. The primary end point, all-cause mortality and non-fatal MI during a median follow-up of 4.6 years, was not different in the two groups (18.5% and 19%; P = .62). There was also no difference in a prespecified composite secondary outcome of death, MI, and stroke, or in hospitalization for acute coronary syndrome or MI. The annual cardiac mortality rate in COURAGE was quite low (0.4%). Approximately a third of patients randomized to medical therapy required revascularization for symptom relief. There was a statistically significant difference in the rates of freedom from angina during most of the follow-up period in favor of PCI, but at 5 years, the rates were similar. This study supported current practice guidelines recommending initial medical therapy for stable angina with the caveat that initial screening with stress testing and/or coronary angiography may be necessary first to detect high-risk patients excluded from COURAGE. In a COURAGE nuclear substudy of 314 patients who had baseline and follow-up myocardial perfusion single-photon emission computed tomography (MPS), there was a greater reduction in ischemia by PCI.52 There was also a significant correlation between the amount of ischemia detected on the follow-up MPS and the occurrence of death or MI (experienced death or MI: no ischemia, 0%; mild ischemia,16%; moderate ischemia, 22%; severe ischemia, 39%; P = .002). These trends are in the same direction as the ACME trial, which reported that ischemia normalization was associated with improved event-free survival in long-term follow-up.53
The Bypass Angioplasty Revascularization Investigation 2 Diabetes (BARI 2D) trial randomly assigned 2368 patients with type 2 diabetes and stable coronary disease to either immediate revascularization or intensive medical therapy.54 Patients had stenosis of at least one major epicardial coronary artery; the method of revascularization (PCI or CABG) was selected by the responsible physician to be the more appropriate therapy for each patient. A total of 1605 patients were selected for the PCI stratum; 798 were assigned to PCI, and 807 were assigned to medical therapy. Primary end points were death and a composite of death, MI, or stroke. Eighty percent of PCI procedures involved single-vessel intervention (56% received a bare-metal stent, 34.7% received a DES, and 9.3% received balloon angioplasty alone). Over an average follow-up of 5.3 years, 43% of the PCI stratum patients assigned to medical therapy underwent clinically indicated revascularization. At 5 years, rates of survival in the PCI stratum did not differ significantly between the PCI and medical therapy groups, and event-free survival was not different. In the PCI stratum, costs were $73,400 for revascularization with PCI versus $67,800 for medical therapy (P < .02).55 The low rates of use of both DESs and thienopyridines (21%) were limitations. The results in the PCI-assigned patients were interpreted by the authors to show that in diabetics with mild to moderate CAD, an initial strategy of optimal medical therapy was preferred over initial PCI given the lack of difference in hard end points on follow-up. The CABG stratum of BARI 2D consisted of 763 diabetic patients who were randomized to CABG or medical therapy. Patients for whom the CABG stratum was prespecified had significantly more three-vessel disease, proximal LAD disease, chronic total occlusions, and prior MIs than patients in the PCI stratum. Although there was no difference in survival between CABG and medical treatment at 5 years, there was a higher event-free survival (survival without MI or stroke) in CABG-treated patients (77.6% vs 69.5%; P = .01). There was also a significant reduction in MI (P = .001) and cardiac death/MI (P = .002) with CABG.56 Although the overall results of the trial were neutral. BARI 2D supports the use of revascularization over medical therapy in diabetic patients with extensive coronary disease similar to that in the CABG stratum but did not directly compare CABG and PCI.
The issue of whether to choose CABG or PCI in patients with multivessel disease must be guided by the long-term outcome of observational and randomized trials comparing bare-metal stents with bypass surgery and by ongoing trials comparing DESs with CABG (see Table 62–1). Follow-up data from the Arterial Revascularization Therapies Study (ARTS), which randomized 1205 multivessel disease patients to bare-metal stent or standard CABG, were reported. At 1, 3, and 5 years, there was no difference in death or MI; however, repeat interventions were higher in the stent group.57-59 One-year survival free of death, MI, and reintervention was seen in 89.4% of the surgical group and in 75.2% of the stent group (P < .04), but at 1 year, costs were higher for surgery (€13,645 vs €10,860). The occurrence of late events in 25% of ARTS stented patients was approximately one half that observed in previous trials using balloon angioplasty. In the smaller Argentine Randomized Study of Stents Versus CABG in Multivessel Disease (ERACI II), 450 patients were randomized, and at 18.5 months, survival was better in the stent group, and freedom from MI was higher, but repeat revascularization was needed more often in the stent group, and costs were similar.60 The Stent or Surgery (SOS) trial, conducted in 53 European and Canadian centers, randomized 500 multivessel disease patients to CABG and 488 patients to bare-metal stent. At 1 year, repeat revascularization was higher with stents.61 The mortality at 5 years favored surgery.62 In the Angina With Extremely Serious Operative Mortality Evaluation (AWESOME) study, 454 high surgical risk patients with refractory ischemia were randomized to PCI (approximately one half received stents) or CABG.63 Survival and symptom relief were similar at 3 years, but repeat revascularization was more frequent in the stent group. At 5 years, in the AWESOME study, average total costs were $63,896 for PCI versus $84,364 for CABG, suggesting that PCI was the economically “dominant” strategy in high-risk patients.64,65 A meta-analysis of 1533 patients from four randomized trials comparing bare-metal stents with CABG (patients from ARTS, SOS, ERACI II, and MASS-II) revealed no difference in MI, death, or stroke, but more repeat revascularizations were needed in stent-treated patients (18% vs 4%; P <.001; Fig. 62–2).66
Figure 62–2.
A meta-analysis of 1-year outcomes of patients from four randomized trials comparing bare-metal stents with coronary bypass surgery (red line represents coronary artery bypass grafting group). MI, myocardial infarction. Reproduced with permission from Mercado et al.66
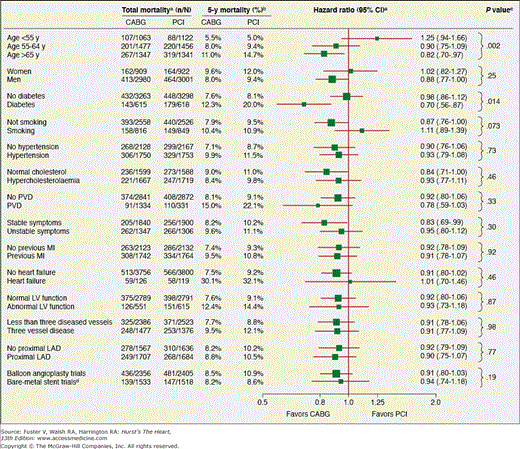
The most recent and authoritative meta-analysis comparing CABG and PCI reported on pooled individual data from almost 8000 multivessel disease patients from 10 randomized trials. This study included 95% of all randomized patients.67 This study concluded that at 6 years, there was no overall difference in survival between PCI and CABG. However, there was a survival benefit with CABG over PCI in diabetics (hazard ratio [HR], 0.70) and in patients age 65 or older (HR, 0.82). Subgroup analyses for mortality are displayed in Fig. 62–3. The finding that patient age modified the relative effectiveness of PCI and CABG on survival had not been reported previously. The generalizability of this data is limited by the fact that patients with extensive three-vessel or left main disease were usually excluded and some patients with mild-moderate disease were appropriately treated with PCI and by the lack of more effective contemporary adjunctive medical therapy and revascularization strategies, including DESs.
Figure 62–3.
Subgroup analyses for mortality after treatment with coronary artery bypass graft (CABG) or percutaneous coronary intervention (PCI). LAD, left anterior descending artery; LV, left ventricular; MI, myocardial infarction; PVD, peripheral vascular disease. The vertical line indicates a hazard ratio of 1.0, equivalent to no difference between treatment groups. a Based on the full duration of follow-up in all trials. b Pooled unadjusted 5-year Kaplan-Meier survival rates. cP value for the treatment by covariate interaction. d The analysis that compares patients enrolled in balloon angioplasty trials and bare-metal stent trials is pooled and not stratified by study. Reproduced with permission from Hlatky et al.67
Data from registries in New York state permitted a comparison of outcomes of 59,314 patients with multivessel disease who were treated with bare-metal stents or CABG between January 1997 and December 2000.68 Patients with prior revascularization, left main disease, or MI within 24 hours were excluded. Patients selected for CABG were older and had more comorbidity, lower ejection fractions, and more severe coronary disease. After adjustment for baseline differences between CABG-treated and stent-treated patients, there was a significantly higher likelihood of survival at 3 years with CABG in all anatomic subgroups. The adjusted survival curves diverged quite early and continued to diverge over the 3-year follow-up period. Repeat revascularization was required in only 4.9% of CABG patients compared with 35.1% of stented patients (P <.001). The weakness of registries lies in the difficulty for adjustments to correct for all baseline differences. The disparity in mortality outcomes between the New York registry and the prior individual, randomized controlled trials fueled the controversy regarding the optimal revascularization strategy for patients with multivessel disease.
DES: A New Dominant Strategy
Although metallic stents conferred a significant advantage over balloon angioplasty both in reducing initial complications and late events, in-stent restenosis remained a major obstacle to wider application of percutaneous revascularization, especially in complex lesions and clinical subsets. Although one systemic drug trial aimed at reducing in-stent restenosis caused by neointimal hyperplasia was positive,69 the most exciting strategy for restenosis prevention was the DES, which combined mechanical scaffolding with local pharmacologic action.47,70-75 The agents that were applied to first-generation DESs were sirolimus, an immunosuppressive macrolide antifungal agent that blocked the G1/S phase of cellular replication, and paclitaxel, an antimicrotubular agent that acted predominantly during the mitosis (M) phase (Fig. 62–4). Both agents decreased smooth muscle migration and proliferation and were shown to be effective in reducing restenosis in clinical trials when polymers were used to bind the agent to the stent. Other agents evaluated were the sirolimus analogs zotarolimus and everolimus, which inhibit the cell cycle at the same point as sirolimus. The longest follow-up is with the sirolimus-eluting stent (SES). Among 45 patients with de novo lesions in vessels of 3.0 to 3.5 mm and lesion length <18 mm, there was no in-stent restenosis or stent thrombosis at 2 years, and ultrasonography at 4 years revealed continued effectiveness.75
Figure 62–4.
Schematic of the cell cycle and its regulatory mechanisms that are relevant for the inhibitory effect imposed by sirolimus (SRL) and paclitaxel (PTX). The cell cycle is regulated by the oscillating activities of cyclin/cyclin-dependent kinase (CDK) complexes. Cyclin-dependent kinase inhibitors (CKIs) negatively control the activity of distinct cyclin/CDK complexes. The CKIs of the Cip/Kip class are major regulators of the cell cycle in its initial stage, the G1/S phase. Cip/Kip CKIs include p21Cip1 and p27Kip1, among others. Both are critical cell cycle regulators in smooth muscle cells. p27Kip1 activity is regulated at the posttranscriptional level via protein stability and translation. Subsequent to binding its intracellular receptor FKBP12 (FK-506 binding protein), SRL inhibits the activity of mammalian target of rapamycin (mTOR). mTOR is a pivotal protein kinase that mediates mitogen-induced cell proliferation. The inhibition of mTOR by SRL attenuates p27Kip1 degradation, thus increasing p27Kip1 protein stability. Additionally, p27Kip1 protein translation may also be enhanced. Non–p27Kip1-dependent mechanisms of mTOR that lead to stimulation of cap-dependent protein synthesis and are inhibited by SRL include p70S6K and eIF4E activation, the latter via induction of eIF4E-binding protein-1 (4EBP1). Paclitaxel impacts predominantly during cell division in the mitosis (M) phase of the cell cycle through centrosomal impairment, induction of abnormal spindles, and suppression of spindle microtubule dynamics. Reproduced with permission from Wessely et al.72
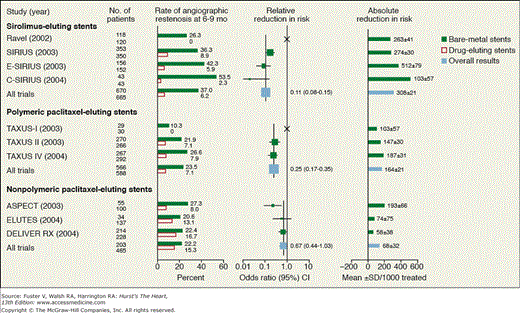
In the Randomized Study With the Sirolimus-Coated Bx Velocity Balloon-Expandable Stent in the Treatment of Patients With De Novo Native Coronary Artery Lesions (RAVEL), 238 patients were randomized to either bare-metal stents or SESs with 6-month angiographic follow-up.76 The mean lesion length was 9.6 mm. No patient in the SES group had restenosis compared with 27% in the bare-metal stent group (P <.001) (Fig. 62–5). At 1 year, major cardiac event rates were 5.8% for the DES group and 20% for patients treated with bare-metal stents (P <.001), a difference entirely attributable to more repeat revascularizations in the bare-metal stent group. This result with relatively simple lesions was not achieved in the subsequent US multicenter Sirolimus-Coated Bx Velocity Balloon-Expanded Stent in the Treatment of Patients With De Novo Coronary Lesions (SIRIUS) trial, where 1058 patients with longer lesions were randomized to receive a sirolimus-coated or bare Bx Velocity (Cordis Corporation, Bridgewater, NJ) stent.77 In-segment restenosis was reduced from 37% to 9% (P <.001). Target lesion revascularization was reduced from 17% to 4% (P <.001), and major cardiac events were reduced from 19% to 7% (P <.001). Based on this favorable safety and efficacy data, the FDA approved the SES for marketing in the United States in 2003. DESs were adopted quite rapidly in the United States compared with Canada and Europe.78 Figure 62–5 displays the results of several additional trials using SESs.
Figure 62–5.
Results of selected trials of drug-eluting stents. Reproduced with permission from Serruys et al.47
Several clinical trials evaluated paclitaxel-eluting stents (PESs) compared with an identical bare-metal stent (TAXUS; Boston Scientific, Natick, MA; see Fig. 62–5). With PES, there was a highly significant reduction in restenosis and a consistent safety profile. In a pooled analysis of 2797 patients enrolled in four TAXUS trials (I, II-SR, IV, and V), at 5 years, a 44% reduction in target vessel revascularization with no difference in death, cardiac death, MI, or major adverse coronary events (MACE) was seen.79 Initial clinical evaluation of a zotarolimus-eluting stent (ZES) (Endeavor; Medtronic, Minneapolis, MN) was performed in the ENDEAVOR I safety trial and in ENDEAVOR II, a 1197-patient randomized comparison to an identical bare-metal stent.80 The mean in-stent late loss was 0.61 mm, significantly higher than in trials of SESs and PESs, but restenosis was reduced from 35% to 13% (P < .00001) with low stent thrombosis (0.5%). Similarly, an everolimus-eluting stent (EES) was compared with a bare-metal stent with significant reductions in restenosis and favorable safety for up to 4 years.81
A varying degree of suppression of intimal hyperplasia noted in observational and randomized trials suggested differences between DESs and led to head-to-head comparative studies. In a 1353-patient randomized multicenter trial, SES and PES were compared.82 The primary end point, binary restenosis at 8 months, was not significantly different (9.6% vs 11.1%; P = .31), and there was no difference in death, cardiac death, MI, target lesion revascularization, or MACE at 8 months. Angiographically determined late loss was less for the SES (0.09 mm vs 0.31 mm; P <.001).
In a two-center, randomized study of 1012 patients, outcomes were better with SESs than with PESs with respect to in-segment restenosis, target vessel revascularization, and late loss, but at 5 years, the gap closed, and there were no differences in these parameters or in death, cardiac death, or MI.83
Second-generation DESs have theoretical advantages over the first generation due to the thin-strut design and reduced polymer thickness resulting in improved stent flexibility, improved deliverability, and better healing. ZESs, which have shorter drug-elution time, more late loss, and better strut coverage than SES or PES at 6 to 8 months, were compared in randomized trials to SESs in ENDEAVOR III and SORT-OUT III and to PESs in ENDEAVOR IV and ZEST (Comparison of the Efficacy and the Safety of Zotarolimus-Eluting Stent Versus Sirolimus-Eluting Stent and Paclitaxel-Eluting Stent for Coronary Lesions).84-87 These studies demonstrated similar clinical efficacy at 1 to 3 years, with improved safety with ZES in some84,85 but not all86,87 studies. A ZES with slower drug elution (A Randomized Comparison of a Zotarolimus-Eluting Stent With an Everolimus-Eluting Stent for Percutaneous Coronary Intervention [RESOLUTE]) is currently undergoing evaluation. EES was compared with PES in SPIRIT II, III, IV, and V and COMPARE (A Randomized Controlled Trial of Everolimus-Eluting Stents and Paclitaxel-Eluting Stents for Coronary Revascularization in Daily Practice).88-91 The second-generation EES outperformed the PES, with an approximate 50% decrease in side branch occlusions, fewer in-hospital and late MIs, and lower repeat revascularization. However, in diabetics, outcomes were similar with EES and PES. Stent thrombosis occurred in <1% of EES-treated patients at 1 year. It is important to note that when second-generation stents were used off-label, MACE and repeat revascularization rates doubled compared with standard use and that registries and postmarket studies will be important to track outcomes after use of these devices.92
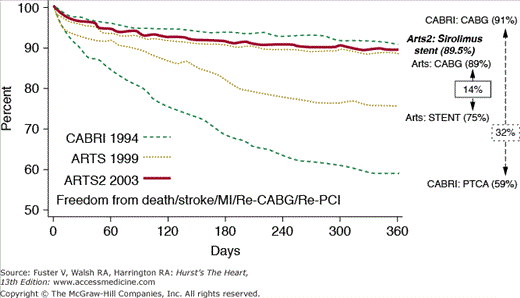
The clear restenosis advantage of DESs compared with bare-metal stents in most lesion and patient subsets has engendered an increasing use of these devices in patients with multivessel disease. However, randomized trial data are only now becoming available to guide the choice between PCI with DESs and CABG in multivessel disease patients. Observational data have been reported from the ARTS II registry, in which 607 multivessel disease patients who received an average of 3.7 SESs were compared with the surgical and bare-metal stent arms of ARTS I.93,94 At 1 year, 89.5% of ARTS II patients were MACE free, a similar outcome to ARTS I CABG patients. Figure 62–6 shows the Kaplan-Meier curves comparing ARTS I and II and CABRI outcomes. However, repeat intervention was needed in only 4.1% of ARTS I CABG patients versus 8.5% (P = .003) of ARTS II drug-eluting patients and 12.6% of diabetics in ARTS II.95
Figure 62–6.
Comparison of outcomes from Coronary Angioplasty Versus Bypass Revascularization Investigation (CABRI) (balloon angioplasty) patients, Arterial Revascularization Therapies Study (ARTS) stent-treated patients, ARTS coronary artery bypass graft (CABG)-treated patients, and ARTS II patients treated with sirolimus-eluting stents. MI, myocardial infarction; PCI, percutaneous coronary intervention; PTCA, percutaneous transluminal coronary angioplasty. Reproduced with permission from Serruys.94
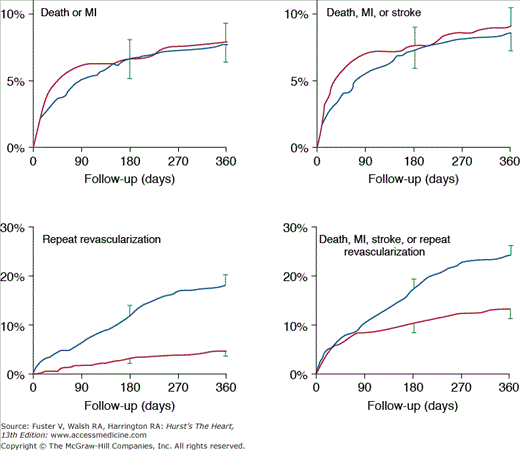
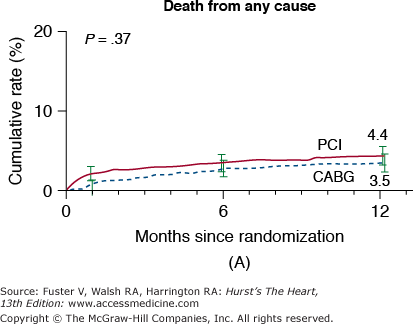
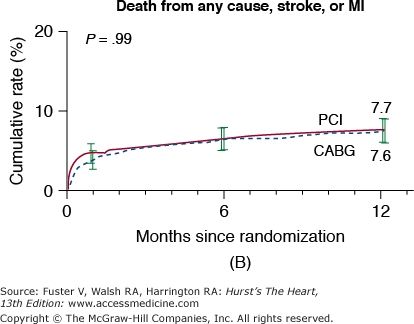
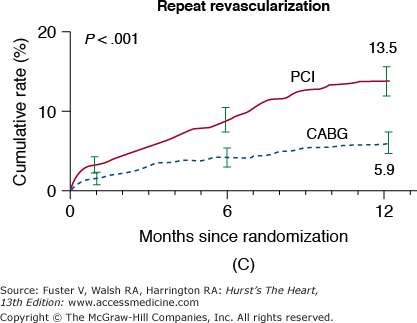
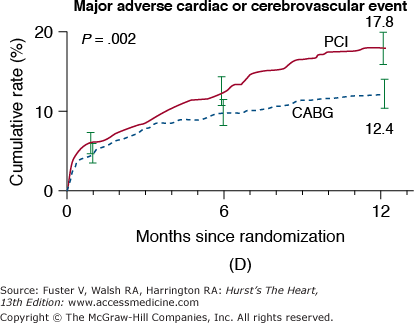
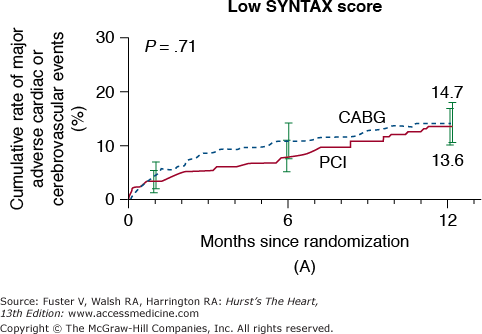
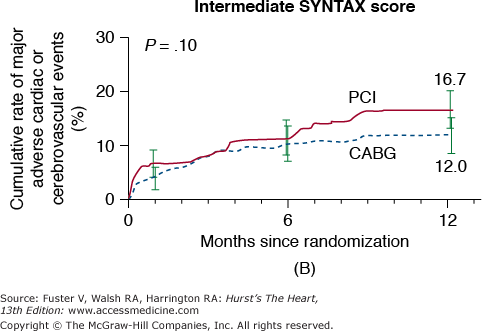
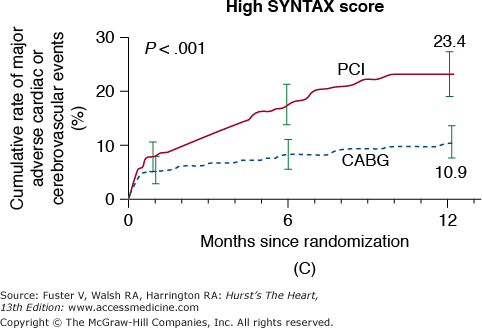
In the New York State Registry study of multivessel disease patients treated with DES or CABG, unadjusted data indicated similar mortality. However, after adjustment for baseline differences, survival was better with CABG for patients with two- or three-vessel disease.96 In the Synergy Between PCI With TAXUS and Cardiac Surgery (SYNTAX) trial, 1800 patients with three-vessel or left main CAD were randomized to CABG or PCI with TAXUS stents.97 This was designed as an “all-comers” study, with >70% of screened patients enrolled in the randomized or registry cohorts. At 1 year, the primary end point (death, MI, stroke, or repeat revascularization, collectively termed major adverse cardiac and cerebrovascular events [MACCE]) occurred more often after PCI compared with CABG (17.8% vs 12.4%; P = .002). However, the rates of death and MI were similar, and stroke was more likely with CABG (2.2% vs 0.6%; P = .003) (Fig. 62–7). The complexity of coronary disease was remarkable, with a mean stented length of >85 mm; the mean number of stents per patient was 4.6 ± 2.3, and 48% of patients received five or more stents.98 Coronary angiograms were scored for severity of disease by the SYNTAX score algorithm.99 The rates of MACCE at 12 months after CABG were not influenced by SYNTAX score (Fig. 62–8). In contrast, MACCE was significantly increased after PCI in patients with high SYNTAX scores (23.4%) compared with intermediate (16.7%) or low scores (14.7%). At 2 years, MACCE rates were equivalent between CABG and PCI in patients with the lowest SYNTAX scores (scores of 0-22, 16.5% vs 21.9%; P = .25) but favored CABG in the intermediate- and high-score groups.100 A similar pattern was seen in patients with three-vessel disease, and it was suggested that the SYNTAX score may be useful in choosing a revascularization strategy for this subgroup (SYNTAX score 0-22, consider PCI; >22, consider CABG).101 In patients with left main coronary disease, 2-year MACCE was similar after CABG and PCI in patients with SYNTAX score <33, which constituted 34% of patients with left main disease in SYNTAX.101 Preliminary findings suggest that the discriminatory value of the purely angiographic SYNTAX score may be improved by addition of clinical factors (clinical SYNTAX score = SYNTAX + age/ejection fraction + 1 if creatinine >2).102 The principal finding of these studies comparing CABG and DES is the superiority of CABG over a PCI approach in patients with the most advanced CAD.
Figure 62–7.
Rates of outcomes among the study patients, according to the treatment group. Kaplan-Meier curves are shown for the percutaneous coronary intervention (PCI) group and the coronary artery bypass grafting (CABG) group for death from any cause (A); death, stroke, or myocardial infarction (MI) (B); repeat revascularization (C); and the composite primary end point of major adverse cardiac or cerebrovascular events (D). The two groups had similar rates of death from any cause (relative risk with PCI vs CABG, 1.24; 95% confidence interval [CI], 0.78-1.98) and rates of death from any cause, stroke, or MI (relative risk with PCI vs CABG, 1.00; 95% CI, 0.72-1.38). In contrast, the rate of repeat revascularization was significantly increased with PCI (relative risk, 2.29; 95% CI, 1.67-3.14), as was the overall rate of major adverse cardiac or cerebrovascular events (relative risk, 1.44; 95% CI, 1.15-1.81). The I bars indicate 1.5 standard errors. Relative risks were calculated from the binary rates. P values were calculated using the χ2 test. Reproduced with permission from Serruys et al.97
Figure 62–8.
Rates of major adverse cardiac or cerebrovascular events among the study patients, according to the treatment group and SYNTAX score category. Kaplan-Meier curves are shown for the percutaneous coronary intervention (PCI) group and the coronary artery bypass grafting (CABG) group for the major adverse cardiac or cerebrovascular events at 12 months. The 12-month event rates were similar between the two treatment groups for patients with low SYNTAX scores (0-22) (A) or intermediate SYNTAX scores (23-32) (B). Among patients with high SYNTAX scores (≥33, indicating the most complex disease), those in the PCI group had a significantly higher event rate at 12 months than those in the CABG group. SYNTAX scores were calculated at the core laboratory. The I bars indicate 1.5 standard errors. P values were calculated using the χ2 test. SYNTAX, Synergy Between Percutaneous Coronary Intervention With Taxus and Cardiac Surgery. Reproduced with permission from Serruys et al.97
The first randomized trial of DES and CABG in multivessel disease in diabetics included 510 patients, 65% of whom had three-vessel disease. In addition to revascularization, patients received aggressive diabetic control.103 DESs were used in 69% of stented patients who received 3.6 stents on average (mean total length of 71 mm). At 1 year, the composite primary end point (death, MI, or stroke) was not different in the PCI and CABG groups (13% vs 11%; P = .39), but the secondary end point (death, MI, stroke, or repeat revascularization) occurred more frequently in the PCI group (19% vs 11%; P = .02). Although this study was underpowered, the outcomes following DES were encouraging. Results of the Future Revascularization Evaluation of Patients With Diabetes Mellitus: Optimal Management of Multivessel Disease (FREEDOM) trial comparing CABG with DES in diabetic patients are awaited.
DESs reduce restenosis and the need for costly repeat revascularization procedures, but this benefit comes at a significantly higher initial price.104 Two reports suggested that DESs were cost effective.105,106 However, multiple factors influence this calculation, including the number of stents required and the effectiveness of the DES in avoiding the need for repeat intervention.106-112 Subgroups in whom a DES is most likely to be cost effective compared with a bare-metal stent include patients with diabetes, long lesions, small vessels, and lesions in left main artery, proximal LAD, grafts, or other sites at high risk of restenosis. As the number of lesions escalates, the cost effectiveness of DESs quickly erodes. The impact of this more expensive new technology is different for the payer, provider (ie, hospital), and vendor.107 Modeling of the ARTS II and ARTS CABG data suggest that the DES had an overall economic advantage.112 However, when long-term dual antiplatelet therapy is added to prevent late stent thrombosis, DESs are likely to be cost effective only in patients at particularly high risk of restenosis.113
Although clinical trials provide compelling evidence suggesting that the use of DESs is safe and highly effective in reducing repeat revascularizations, recent studies reporting coronary endothelial dysfunction, coronary spasm, hypersensitivity reactions, stent fracture or malapposition, delayed healing, and late stent thrombosis, which are complications not reported with bare-metal stents, are worrisome.114-128 In addition, the increased risk associated with nonresponsiveness to129,130 or premature discontinuation of antiplatelet therapy, with a reported HR as high as 89.78,121 may be unavoidable because of complications from required surgery or bleeding. Advanced age, lower socioeconomic status, and inadequate counseling are also associated factors in patients who prematurely omit dual antiplatelet therapy. Although a step forward in treating patients with CAD, DESs are not a panacea and should be used prudently rather than reflexively during PCI. Newer generations of DESs may well further improve the risk-benefit profile of this technology, but this assessment will require further clinical studies.
Adjunctive Strategies
The second-generation thienopyridine clopidogrel is a prodrug whose active metabolite inhibits platelet activation by irreversibly blocking the adenosine diphosphate (ADP) receptor (P2Y12). It has better tolerability and fewer adverse effects and is at least as effective as ticlopidine; along with aspirin, it is commonly administered prior to stent implantation. Evidence supports its use in nonstent PCI as well.131,132 Clopidogrel loading doses of 600 mg are needed to produce potent inhibition of ADP-induced platelet aggregation within 2 hours.133-135 A 300-mg loading dose is commonly used when longer pretreatment is possible and has been shown to produce maximal platelet inhibition within 24 hours with substantial inhibition at 15 hours.136 A 600-mg loading dose of clopidogrel 2 hours before elective, low-risk stent implantation showed similar results to those obtained with the same dose of clopidogrel plus routine abciximab administration.137 When tested in high-risk patients with non–ST-segment elevation acute coronary syndrome, the clopidogrel 600-mg loading dose alone was also as effective as clopidogrel plus abciximab in troponin-negative patients, but not in troponin-positive patients (adverse events: 18.3% with clopidogrel alone vs 13.1% with clopidogrel plus abciximab; P = .02).138 After PCI, long-term (1 year) clopidogrel was associated with a 27% relative reduction in adverse ischemic events (P = .02) compared with 4 weeks of therapy.139 These findings extended and amplified the similar findings of the Percutaneous Coronary Intervention–Clopidogrel in Unstable Angina to Prevent Recurrent Events (PCI-CURE) trial.132 Major bleeding was not significantly increased at 1 year, and clopidogrel therapy for 1 year was highly cost effective.140
Recent reports suggest that inadequate inhibition of platelet aggregation may occur in patients with higher body mass index141 and that insensitivity to clopidogrel is more common than previously thought.130 Both factors may contribute to periprocedural ischemic complications and stent thrombosis. Depending on the definition used, 10% to 15% of patients undergoing PCI are resistant to aspirin, and up to 25% are resistant to clopidogrel. In addition, approximately half of aspirin-resistant patients have a lower response to clopidogrel, placing them at higher risk of periprocedural myonecrosis and stent thrombosis.142 Reliable, standardized, bedside measures of resistance to dual antiplatelet therapy are not routinely available, and there is no clear clinical trial evidence to guide selection of alternative antithrombotic strategies among patients with an insufficient response to either aspirin or clopidogrel treatment. Duration of dual antiplatelet therapy also has not been standardized, but US and European guidelines and practice trends support extension to 12 months after implantation of DESs and after bare-metal stents in patients who do not have a high risk of bleeding.49,143 The heightened risk of thrombosis of a DES associated with premature discontinuation of dual antiplatelet therapy, especially in the setting of noncardiac surgery, deserves emphasizing.121,122
In late 2009, concern surfaced regarding drug interactions between proton pump inhibitors and clopidogrel. Although considerable data suggested that this was not a clinical concern, an FDA warning was issued, and caution was advised in the 2009 guideline statement.49 Three solutions were suggested: (1) using an H2 antagonist; (2) using pantoprazole, a proton pump inhibitor without inhibition effects; (3) and staggering the dosing of medication.144 More definitive data from randomized clinical trials are needed to guide concomitant drug therapies.
Prasugrel, a new thienopyridine prodrug with more rapid and consistent platelet inhibition, was shown in TRITON-TIMI 38 (Trial to Assess Improvement in Therapeutic Outcomes by Optimizing Platelet Inhibition With Prasugrel Thrombolysis in Myocardial Infarction 38) to have a more favorable net clinical benefit end point (mortality, ischemic events, and major bleeding) than clopidogrel in acute coronary syndromes despite increased Thrombolysis In Myocardial Infarction (TIMI) major bleeding.145 Prasugrel was approved by the FDA in July 2009, which cautioned against its use in patients with a history of transient ischemic attack, stroke, and active bleeding and in patients >75 years of age because of increased bleeding risk. Despite increased medication costs (prasugrel = $5.45 per day; clopidogrel = $4.62 per day), prasugrel was shown to be an economically dominant strategy compared with clopidogrel.146,147 Prasugrel has a Class I recommendation (Level of Evidence: B) in the current PCI guidelines.49 A new reversible, direct P2Y12 inhibitor, ticagrelor, which is not yet FDA approved, was also shown to be more effective than clopidogrel when given with aspirin among a broad group of ACS patients, whereas the ultra–short-acting intravenous ADP blocker cangrelor was not superior to either placebo or clopidogrel when studied in a group of patients undergoing PCI.148
The decision to use a glycoprotein (GP) IIb/IIIa platelet receptor inhibitor in the era of high-dose thienopyridine pretreatment is complex and requires an assessment of the patient’s risk of bleeding and ischemic complications with and without these agents. The optimal application of these agents in patients with acute coronary syndromes has not been fully established. The 2007 ACC/AHA guidelines for the management of patients with unstable angina/non–ST-segment elevation myocardial infarction recommend that patients with definite or likely unstable angina/non–ST-segment elevation myocardial infarction selected for an invasive approach receive acetylsalicylic acid (ASA) and either clopidogrel or a GPIIb/IIIa receptor antagonist before angiography (Class I, Level of Evidence: A). They go on to say that it is reasonable to initiate both clopidogrel and a GPIIb/IIIa receptor antagonist in patients with high-risk features or recurrent ischemic discomfort (Class IIa, Level of Evidence: B). The 2007 European Society of Cardiology guidelines recommend early dual antiplatelet therapy with ASA and clopidogrel (Class I), with the addition of a GPIIb/IIIa receptor antagonist for patients with the specific high-risk features of an elevated troponin level, ST-segment depression, or diabetes (Class IIa).138,149 The optimal timing of administration of GPIIb/IIIa therapy is uncertain. The recently published Early Glycoprotein IIb/IIIa Inhibition in Non–ST-Segment Elevation Acute Coronary Syndrome (EARLY ACS) trial found no significant benefit comparing early routine versus delayed provisional eptifibatide.150 The 2009 PCI guidelines give cautious support to the use of early GPIIb/IIIa therapy in patients judged to be at high risk of thrombotic events and low risk of bleeding but say “at this time a high-risk group that would clearly benefit from the early administration of eptifibatide upstream before cardiac catheterization has not been indentified.”49 The use of GPIIb/IIIa antagonists with prasugrel has not been adequately studied.
Unfractionated heparin (UFH) was the thrombin inhibitor used in the first 2 decades of PCI. The appearance of low molecular weight heparin (LMWH) as an acceptable agent in ACC/AHA unstable angina guidelines (a Class IIA recommendation) and FDA approval of three direct thrombin inhibitors (DTIs) for use in PCI challenged the interventional cardiologist to provide optimal antithrombotic therapy for a diverse population of complex patients. Clearly, UFH has a number of disadvantages, including nonlinear anticoagulant kinetics, a requirement for cofactor antithrombin III, an inability to inactivate clot-bound thrombin, platelet activation, stimulation of antibody formation, and a prothrombotic rebound phenomenon. It was generally recommended that anticoagulation for coronary angioplasty procedures with UFH be carried out with a weight-adjusted bolus of 70 to 100 IU/kg without GPIIb/IIIa inhibitors and 50 to 70 IU/kg with GPIIb/IIIa inhibitors. Monitoring of activated clotting time (ACT) was recommended to achieve an ACT target of approximately 300 seconds without GPIIb/IIIa inhibitors and 200 to 250 seconds when these agents were used. Several investigators have suggested that ACT targets with stent implantation can safely be reduced to 200 to 250 seconds to minimize hemorrhagic risks.151,152 Postprocedural heparin infusions were generally not recommended because of increased bleeding.
LMWH inactivates thrombin less than UFH and binds factor Xa more avidly with anti-IIa to anti-Xa ratios that vary from 1:2 to 1:4, depending on the LMWH agent used. LMWH is more bioavailable than UFH, is less inhibited by platelet factor-4, and causes less thrombocytopenia, and its predictable anticoagulant effect eliminates, to a degree, the need for laboratory monitoring. The absence of easy monitoring of LMWH activity has been a stumbling block to broad acceptance of LMWH during PCI by interventionalists accustomed to readily available ACT monitoring of UFH. The ideal LMWH regimen for PCI has not been determined, although achieving an anti-Xa level of >0.5 IU/mL has been a suggested target.153,154 This was achieved in >97% of 242 patients with a 0.5 mg/kg intravenous bolus of enoxaparin, and PCI safety was preserved.155 In a meta-analysis of randomized studies comparing LMWH and UFH in patients undergoing PCI, there was no difference in ischemic events and a nonsignificant trend toward less major bleeding with LMWH.156 The Safety and Efficacy of Enoxaparin in Percutaneous Coronary Intervention (STEEPLE) trial compared the safety of intravenous enoxaparin with UFH in real-world elective PCI with DESs, frequent GPIIb/IIIa inhibitors (40%), and clopidogrel (94%). The trial noted a >50% reduction in major bleeding with enoxaparin (2.8% to 1.2%; P <.007).157 Predictors of bleeding were use of GPIIb/IIIa inhibitors, age ≥75 years, and female sex. The study was underpowered for assessing ischemic events. The Class IIa indication for LMWH as a reasonable alternative to UFH appears warranted pending further investigation.
The DTI bivalirudin was approved by the FDA in 2000 for high-risk PCI, whereas hirudin, argatroban, and lepirudin are all FDA approved as anticoagulants only for use among patients with heparin-induced thrombocytopenia. Advantages of DTIs for PCI include the ease of monitoring their action with ACT measurement, their ability to inactivate clot-bound thrombin, and a favorable safety profile. In 4098 unstable angina patients randomized to UFH or bivalirudin, similar outcomes were reported on initial analysis, but a 22% reduction (P = .039) in 7-day ischemic end points on reanalysis of isoenzyme samples and reduced bleeding were noted with bivalirudin.158,159 In a more contemporary, stent-based PCI experience, the Randomized Evaluation of Percutaneous Coronary Intervention Linking Angiomax to Reduced Clinical Events (REPLACE-I) trial, bivalirudin use reduced adverse events and major bleeding compared to UFH.160 In REPLACE-II, bivalirudin was compared with UFH plus abciximab and was found not to be inferior to the more expensive alternative strategy.161 In the Acute Catheterization and Urgent Intervention Triage Strategy Trial (ACUITY PCI), use of UFH plus a GPIIb/IIIa inhibitor (n = 2561) was compared with bivalirudin plus a GPIIb/IIIa inhibitor (n = 2609) and bivalirudin alone (n = 2619) in treatment of patients with acute coronary syndrome undergoing PCI.162 At 30 days, adverse events (death, MI, ischemic revascularization, or major bleeding) occurred in 11.7% of patients receiving bivalirudin, 15.1% receiving bivalirudin plus GPIIb/IIIa inhibitor, and 13.5% receiving UFH plus GPIIb/IIIa inhibitor (P = .001). This outcome was driven by more bleeding with GPIIb/IIIa inhibitors. An ischemia-related event rate of 8.4% occurred with UFH plus GPIIb/IIIa inhibitors, 8.9% with bivalirudin monotherapy, and 9.4% with bivalirudin plus GPIIb/IIIa. These differences were not significant. In the latest guideline statement, bivalirudin has a Class I indication. These studies suggest that DTIs (primarily bivalirudin) administered during PCI are as effective in avoiding ischemic complications as UFH or UFH plus GPIIb/IIIa in certain patient subsets but with less bleeding risk. It has been suggested that optimal antithrombin therapy for PCI in certain complex patient subgroups, such as those with a high risk of bleeding and heparin-induced thrombocytopenia, is perhaps best accomplished with a DTI, whereas patients with troponin positivity, diabetes, thrombus-containing lesions, and ST-segment elevation infarction are probably best managed with an indirect thrombin inhibitor and GPIIb/IIIa platelet receptor inhibitor.163,164 Further studies are needed to develop clinically relevant risk-benefit scoring systems to better personalize antithrombotic therapy.165
At the time of PCI, or shortly thereafter, administration of a variety of therapeutic agents not directed at the clotting cascade or platelets is associated with improved patient outcomes. Preprocedural high-dose statin therapy is associated with a reduction in periprocedure myocardial injury166 and improvement in survival at 30 days, 6 months, and 1 year after stenting, independent of patient characteristics.167,168 Similarly, β-blocker therapy at the time of elective PCI is associated with reduced post-PCI MI169 and with a 1-year survival benefit (3.9% vs 6.0%; P = .0014) independent of ventricular function, diabetic status, hypertension, or history of MI.170
Although coronary angiography is the reference standard for the diagnosis of CAD, it has major limitations. Assessment of the significance of intermediate or indeterminant lesions, plaque characterization, recognition of diffuse intimal thickening, and accurate assessment of vessel dimensions and lesion extent are important pre-PCI determinations in which intravascular ultrasound (IVUS) greatly surpasses angiography. A minimal lumen cross-sectional area between 3.0 and 4.0 mm2 in major epicardial vessels, excluding left main, has a sensitivity and specificity exceeding 80% for predicting ischemia, whereas a left main minimal lumen diameter of 2.8 mm or minimal lumen area of 5.9 mm2 indicates physiologic significance.171-173 Because stent underexpansion is the most common mechanism leading to failure in the DES era, IVUS can play an important role in identifying the calcified lesion best prepared with the use of rotational atherectomy and ensuring optimal DES expansion, as well as stent apposition to the vessel wall and full lesion coverage.174 It has been suggested that routine IVUS imaging during DES implantation is indicated in a number of high-risk patient subsets (eg, renal failure, limitations to dual antiplatelet use, diabetes, poor left ventricular function) and high-risk lesion subsets (eg, left main, bifurcations, ostial site, small vessels, long lesions, in-stent restenoses). In the authors’ experience, clarification of the cause of all DES failures, either restenosis or stent thrombosis, by use of IVUS is essential to guide treatment decisions regarding further expansion of the underdeployed DES or dilation and additional DESs for restenosis related to intimal hyperplasia. IVUS permits the identification of late stent malapposition (Fig. 62–9), a finding reported to occur in approximately 10% of DESs overall, but more frequently in acute MI (32%) and chronic total occlusions (27%).174,175 In addition, IVUS has identified DES strut fracture as an important mechanism for the development of focal in-DES restenosis, accounting for approximately a third of these lesions.176 Newly developed virtual histology IVUS, permitting classification of tissue into one of four phenotypes (fibrous, fibrofatty, necrotic core, or calcium), is a new addition to the diagnostic armamentarium of the interventionalist and is currently being studied.
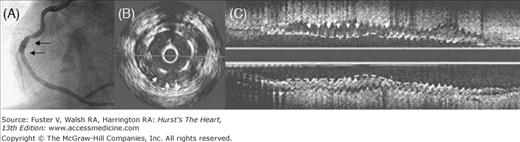
Figure 62–9.
Coronary angiogram of the right coronary artery showing excellent patency (A). Cross-sectional (B) and longitudinal (C) views of intravascular ultrasound indicate that the Cypher stent was not well apposed to the vessel wall. This was not an example of poor stent expansion, however, because this study was performed 18 months after implantation and earlier intravascular ultrasound (IVUS) studies showed good apposition. This is an example of late stent malapposition, which according to Hong et al,125 rarely leads to adverse cardiac events. However, this patient at 40 months after stent implantation developed stent thrombosis and had further increase in malapposition compared with the IVUS study at 18 months. Reproduced with permission from Feres et al.126
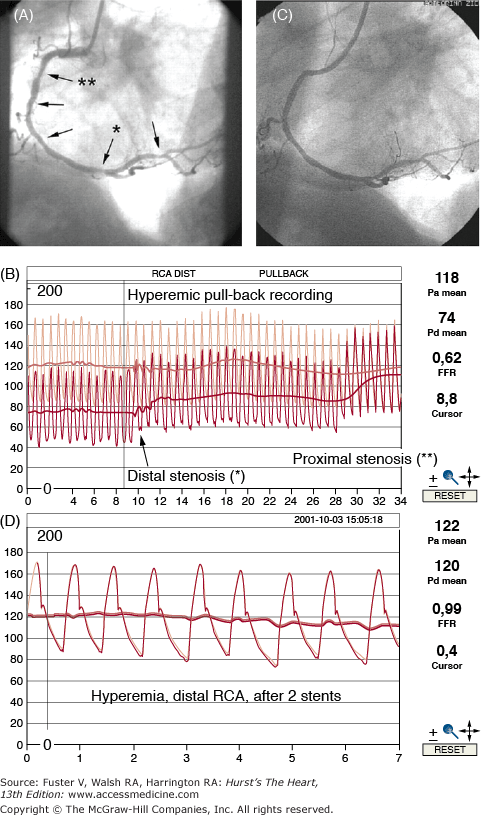
Although intracoronary pressure gradients were used to evaluate lesions in the earliest days of PCI, it was the combination of miniaturized pressure sensors and validation of fractional flow reserve (FFR) measurements that permitted simple, reliable, and reproducible guidewire-based physiologic assessment of lesion severity during coronary interventional procedures177 and provided the ability to determine coronary stenosis severity independent of baseline hemodynamics, blood pressure, and heart rate. An FFR of <0.75 was shown to be 100% specific, 88% sensitive, and 93% accurate in predicting ischemia,178 and it was safe to defer PCI when FFR was ≥0.75.179 The 5-year risk of death or MI in patients with a normal FFR was <1% annually. This easily performed measurement of distal coronary and aortic pressure during maximal hyperemia allows one to also determine culprit stenoses in patients with multivessel disease and to evaluate ostial lesions, left main coronary lesions, serial lesions, and side branch stenoses that may be ambiguous angiographically180 (Fig. 62–10). In the era of costly DESs, FFR can be used to limit intervention only to flow-limiting lesions. In the FFR Versus Angiography for Multivessel Evaluation (FAME) Trial, 1005 multivessel disease patients with stenoses ≥50% underwent PCI guided by standard angiography or FFR (lesions with FFR <0.80 were stented).181 Compared with the angiography-PCI group, FFR-PCI patients consumed fewer resources and had better outcomes at 1 and 2 years.182 Recent state-of-the-art papers nicely summarize the use of coronary physiology in PCI.183,184 The use of FFR to clarify ambiguous anatomy or functional studies has a Class IIa (Level of Evidence: A) indication in the most recent PCI guidelines.49