Classic Blalock-Taussig Shunt
The Blalock-Taussig shunt (
Table 75.2) was the first aortopulmonary shunt; it was first performed in 1944 by Alfred Blalock of the Johns Hopkins University Medical Center. The classic Blalock-Taussig shunt is a direct end-to-side anastomosis of the transected subclavian artery to the pulmonary artery. Legend has it that Helen Taussig, the pediatric cardiologist at Johns Hopkins University, observed that the condition of infants and children with severe forms of tetralogy of Fallot worsened after their patent ductus arteriosus closed. She traveled to Boston and asked Robert Gross if he could create an artificial ductus in these children. He supposedly told her that he was in the business of closing the ductus, not creating new ones. She then met with Blalock, the chairman of the department of surgery at her institution. Blalock had successfully created a left subclavian artery-to-pulmonary artery anastomosis 6 years earlier while developing a canine model of pulmonary hypertension at Vanderbilt University. Taussig suggested to Blalock that he will apply his experimental procedure to the many patients that she saw in her clinic who had cyanosis from insufficient pulmonary blood flow. The first operation was performed on November 29, 1944, on a 15-month-old girl with the diagnosis of tetralogy of Fallot and severe pulmonary stenosis. After that first successful case, hundreds of cyanotic children went to Baltimore for “the operation.” Within the next 2 years, more than 500 patients had undergone the Blalock-Taussig shunt. During this time, there was an early mortality of 16%, and 6% of the patients were considered inoperable. This procedure was very commonly used until the introduction of the modified Blalock-Taussig shunt, which uses an interposition graft of Gore-Tex, avoiding sacrifice of the subclavian artery. The Hopkins group recently reported a six decade experience with 2,000 Blalock-Taussig shunts.
The anastomosis for the classic Blalock-Taussig shunt is classically constructed on the side opposite the aortic arch. When there is a left aortic arch, there is typically a right innominate artery, and using the subclavian artery on this side provides a gentle curve down to the pulmonary artery. With a right aortic arch and mirror-image branching, the left innominate artery provides a similar gentle curve to the pulmonary artery. In contrast, the other subclavian artery for each arch would require an angulation of the artery of nearly 180 degrees to effect the anastomosis.
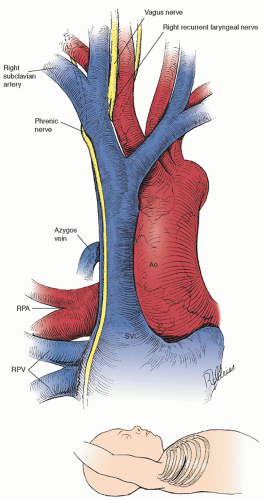
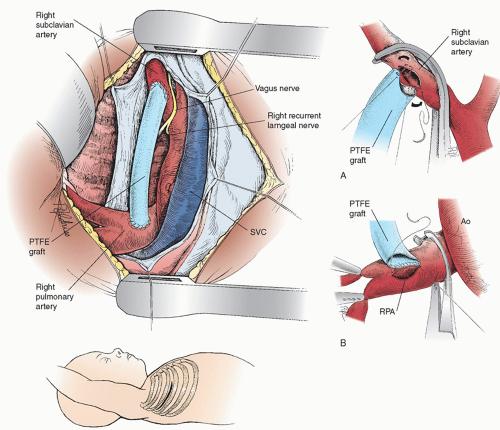
Figure 75.1 illustrates the anatomy for exposure of the subclavian and pulmonary arteries through a right thoracotomy (see inset). The branches of the right subclavian artery are ligated and divided along with the distal subclavian artery, and the subclavian artery is pulled through the loop formed by the right recurrent laryngeal nerve. The carotid artery is freed to provide more mobility. The end of the subclavian artery is spatulated, so the anastomosis is 1.5 to 2.0 times larger than the subclavian artery per se. The anastomosis is constructed to the proximal pulmonary artery, with care taken to avoid the complication of performing the anastomosis to the right upper lobe branch. The subclavian artery will then be in a groove just posterior to the superior vena cava (SVC) and the phrenic nerve (
Fig. 75.2).
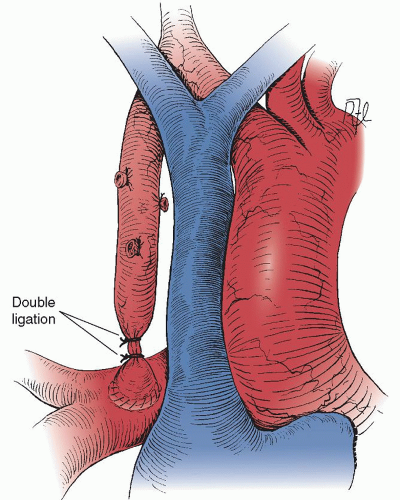
The classic Blalock-Taussig shunt does not require prosthetic material and provides a precise amount of pulmonary blood flow limited by the orifice of the subclavian artery. In addition, the shunt grows with the patient, providing more pulmonary blood flow as the child grows. However, the Blalock-Taussig shunt sacrifices the subclavian artery, which in a small number of cases can result in hand or arm ischemia. In addition, the affected arm is usually shorter than the contralateral arm, is always somewhat cool to the touch, and will not have a palpable pulse. Finally, even with mobilization of the carotid artery and division of the inferior pulmonary ligament, the subclavian artery may still be so short as to cause the pulmonary artery to be “pulled” up and kinked. Takedown of the classic Blalock-Taussig shunt at the time of complete correction through a median sternotomy involves dissection posterior to the SVC. The artery can then be encircled and double ligation performed (
Fig. 75.3).
Modified Blalock-Taussig Shunt
The use of a polytetrafluoroethylene (PTFE) tube for an aortopulmonary shunt was first reported by Gazzaniga and associates in 1976. Three infants with pulmonary atresia underwent aorta-to-pulmonary artery shunt using an interposed 4-mm PTFE tube. DeLeval coined the term modified Blalock-Taussig shunt when he reported on 99 patients operated on between 1975 and 1979 having a prosthesis of Dacron (13) or PTFE (86) interposed between the subclavian and pulmonary arteries (
Table 75.3). Shunt failure rate was 6%, and mortality was 8%. The advantages of the modified Blalock-Taussig shunt,
which has now become the shunt of choice at most congenital heart surgery centers, include (1) preservation of the circulation to the affected arm, (2) regulation of the shunt flow by the size of the systemic (subclavian or innominate) artery, (3) high early patency rate with minimal tissue ingrowth of even a small-diameter expanded PTFE arterial prosthesis (Gore-Tex; W.L. Gore & Associates, Inc., Flagstaff, AZ), and (4) guarantee of adequate shunt length. One disadvantage of the modified Blalock-Taussig shunt is the occasional excessive leaking of serous fluid through the interstices of the fabric of the PTFE. This may result in excessive and prolonged chest tube drainage, localized seroma formation around the graft, or both. This complication occurs in 3% to 5% of patients.
The modified Blalock-Taussig shunt can be performed through a right or left thoracotomy or a median sternotomy. In the last 10 years, we have exclusively used a median sternotomy approach. This facilitates the use of CPB support if the child has significant oxygen desaturation during the procedure. This, of course, is much easier with a median sternotomy approach. CPB should always be readily available for such an instance when a shunt is performed. There are several other advantages to the median sternotomy approach. A patent ductus arteriosus can be ligated as soon as the shunt is opened or when the patient is placed on CPB. The lungs are not compressed by the exposure through a thoracotomy (which can affect the O
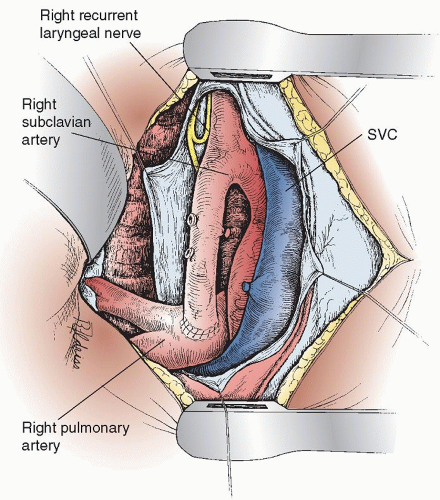
2 saturations). If a thoracotomy approach is used, the side selected depends on the subclavian and pulmonary artery anatomy, the presence and location of a ductus arteriosus, and the great-vessel relationship. The disadvantages (relatively minor) of the median sternotomy are the adhesions with reoperation. With a thoracotomy approach the latissimus dorsi muscle is divided, the serratus anterior is mobilized and spared. The thorax is entered through the fourth interspace. The lung is retracted anteriorly and inferiorly (
Fig. 75.4). The mediastinal pleura is opened posterior to the SVC and phrenic nerve. The azygos vein is doubly ligated and divided. The subclavian artery is encircled with a vessel loop, with care being taken to avoid the right recurrent laryngeal nerve, which passes around the distal innominate artery at the takeoff of the subclavian and common carotid arteries. The right pulmonary artery is dissected free, with care being taken to identify the right upper lobe branch and main pulmonary artery continuation to the right lower and middle lobes. The right upper lobe and the distal right pulmonary artery branches are encircled with vessel loops, which can then be occluded with small Rommel tourniquets. Alternatively, a small vascular clamp can be placed on the pulmonary artery. The patient is administered 1 mg/kg of heparin intravenously.
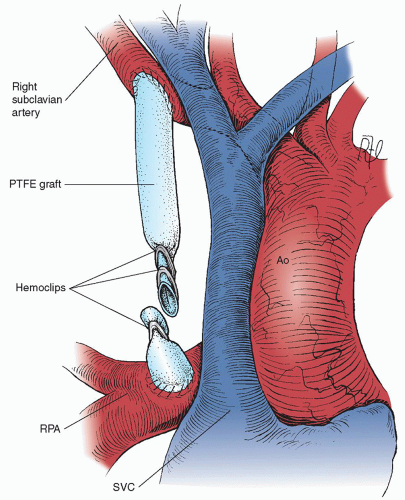
The size of the PTFE graft selected is based on the size of the patient. We currently
use only the heparin-bonded “stretch” PTFE. We use a 3.0-mm shunt for neonates <2.0 kg, a 3.5-mm shunt for neonates 2.0 to 4.0 kg, a 4.0-mm shunt for infants >4 kg, and only rarely a 5-mm shunt in an infant over 5 kg. Nearly all neonates having a shunt procedure will have a bidirectional cavopulmonary anastomosis or a complete repair by 6 months of age; so long-term patency is not an issue. The PTFE is cut to size before the clamps are placed; the clamps distort the relative distance between the subclavian and pulmonary arteries. The graft is usually cut to give it a gentle curve, which allows for some patient somatic growth. It should not kink or distort the pulmonary artery, which can easily happen if the length is not accurate. The vessel loop is used to pull the subclavian artery, and a segment of the vessel is then occluded with a small Castaneda clamp. The PTFE graft is beveled as illustrated in
Fig. 75.4A, and an arteriotomy is created in the inferior aspect of the subclavian artery. The PTFE graft is anastomosed to the opening in the subclavian artery with 7-0 polypropylene suture and a parachute technique. The clamp on the subclavian artery is left in place until the pulmonary artery anastomosis is completed. Repositioning the clamp to the
PTFE graft may increase the risk of blood stasis and shunt thrombosis. The proximal pulmonary artery is controlled with another small Castaneda clamp. The Rommel tourniquets are snugged on the right upper and right distal pulmonary arteries. A longitudinal arteriotomy is created in the superior aspect of the right pulmonary artery. With a single 7-0 polypropylene suture, the PTFE graft is anastomosed to the pulmonary artery (
Fig. 75.4B). The distal Rommel tourniquets are released first, then the clamps on the pulmonary and the subclavian arteries.
There should be a nearly instantaneous rise of approximately 10% to 15% in the patient’s oxygen saturation as monitored by pulse oximetry. A thrill should be palpable in the shunt and in the distal pulmonary artery. It is important to maintain adequate systemic arterial pressure before, during, and after the operation to prevent early shunt thrombosis. This may require the use of inotropic support (dopamine, dobutamine). Bleeding is controlled with absorbable gelatin sponge (Gelfoam) soaked with topical thrombin. The heparin is not routinely reversed with protamine unless there is excessive bleeding from the suture lines. The graft should lie in a groove posterior to the SVC, where it is accessible at the time of takedown for intracardiac repair through a median sternotomy approach. The chest is closed in layers with a single chest tube directed posteriorly and superiorly. We routinely sedate and ventilate the child for the first 24 hours postoperatively.
Williams and colleagues recently reviewed 2,000 shunt procedures performed at the Johns Hopkins hospital since Alfred Blalock’s first operation. Of these patients, 70% carried a diagnosis of tetralogy of Fallot as a result of the large number of cases done in the 1940s and 1950s before the advent of complete intracardiac repair. Operative mortality ranged from 15% in the first decade of use (1940s) to 8% to 10% in the modern era. Complications of stroke, bleeding, and infection are all <5% in the current era. Over the six decades, the review encompassed its use decreased, the operative mortality declined, and there was a shift in application to single ventricle patients.
Petrucci reviewed outcomes of all shunt procedures recorded in the Society of Thoracic Surgeons Congenital Heart surgery database during the modern era (2002 to 2009). This study included 1,273 patients. The overall mortality rate was 7.2% and the complication rate (need for mechanical circulatory support or unplanned reoperation) was 13%. Low weight (<3 kg), functionally univentricular hearts, or a diagnosis of pulmonary atresia with intact ventricular septum were all risk factors for death. Notably, one-third of deaths occurred within the first 24 hours of operation.
The takedown at the time of intracardiac repair through a median sternotomy is quite easily performed, particularly if the shunt was placed on the right side (
Fig. 75.5). The shunt can be identified by dissecting the medial aspect of the SVC posteriorly. Locating a shunt on the left side is more difficult, but it can be identified either by dissecting along the left pulmonary artery, along the aorta to the shunt, or by entering the pleural space and approaching the shunt laterally. The dissection will first encounter a thick fibrous “peel,” which typically forms around the PTFE graft. After the plane between the “peel” and the PTFE graft is entered, the dissection proceeds relatively quickly and easily and enough graft length can be achieved for double hemoclip application proximally and single hemoclip distally. The graft should be divided between the hemoclips to prevent distortion of either the subclavian artery or the pulmonary artery as the patient grows and the distance between these two vessels naturally increases. No attempt is made to remove the proximal portion of the graft. In some patients, the distal graft will require removal to facilitate performing a bidirectional Glenn anastomosis. However, if the operation involves repair at the site of the main pulmonary artery, the distal graft can be left in place and typically does not cause a residual peripheral pulmonary artery stenosis.
As mentioned earlier, our preference is to perform the modified Blalock-Taussig shunt through a median sternotomy approach. This is particularly useful for the child who is having uncontrollable episodes of oxygen desaturation despite sedation, paralysis and full ventilation, and administration of phenylephrine (Neo-Synephrine). CPB with a single atrial cannula and cooling to 32°C will allow the Blalock-Taussig shunt to be performed in a safe and unhurried
fashion, with the heart beating in normal sinus rhythm throughout the procedure. We and others have adopted the median sternotomy as the standard approach for a modified Blalock-Taussig shunt, believing that the safety factors outweigh the adhesions at reoperation.
One other consideration at the time of Blalock-Taussig shunt construction is the question of whether or not to ligate the patent ductus arteriosus. Our practice until recently has been to ligate the patent ductus arteriosus either when CPB is started or when the clamps for the shunt are released (no CPB). A recent review by Zahorec and colleagues noted a higher early hospital mortality (9.7% vs. 0%) when the arterial duct was surgically closed versus being left open (n = 62).
One other trend in the management strategy of neonates requiring a source of pulmonary blood flow is the use of transcatheter ductal stenting. McMullan and colleagues recently reported 11 neonates who underwent percutaneous transcatheter placement of an endovascular stent to maintain patency of the arterial duct as an alternative to modified Blalock-Taussig shunt. When compared with 43 patients who had a modified Blalock-Taussig shunt during the same time period, they found that percutaneous stenting had greater freedom from reintervention and fewer procedural complications. These promising results merit consideration as world-wide experience with this new technique expands. Continued work with this alternative is necessary prior to widespread adoption of this new transcatheter approach.