Obstructive Lung Disease
Obstructive lung diseases are characterized by progressive expiratory airway flow limitation and respiratory symptoms, including chronic cough, sputum production, and dyspnea. These diseases are common and associated with significant morbidity and mortality. Clinically, asthma is characterized by reversible airflow obstruction, chronic bronchitis by a productive cough, and emphysema by irreversible airflow obstruction, dyspnea on exertion, pulmonary hyperinflation, and destruction of alveolar walls. Pathologically, asthma is characterized by inflammation and airway remodeling, whereas chronic obstructive pulmonary disease (COPD) is categorized by emphysema, small airway inflammation and fibrosis, and mucous gland hyperplasia (1).
Although there are many similarities among the major pathophysiologic categories of disease causing chronic airflow obstruction, the advent of new therapeutic modalities and differences in patient prognosis highlight the importance of making a specific diagnosis. For example, patients with nonasthmatic airflow obstruction have a greater rate of decline in forced expiratory volume in 1 second (FEV1) and poorer survival than patients with chronic asthmatic bronchitis (2), and patients with a self-reported history of chronic bronchitis have a steeper drop in FEV1 than patients with asthma (3). By using clinical history, physical examination, and pulmonary function testing together with imaging, in particular high resolution computed tomography (HRCT), the subsets of chronic obstructive lung disease can be distinguished and therapy correctly tailored.
Clinically and radiographically, manifestations of more than one form of COPD may be present in the same patient.
Table 15.1 lists the common diseases that are traditionally considered under the heading “COPD.” COPD is currently defined as a disease state characterized by airflow obstruction that does not change markedly over months of observation. It is usually pathologically a result of chronic bronchitis or emphysema (1,4). According to a National Heart, Lung and Blood Institute Workshop on the subject, “The term ‘COPD’ is generally used in
clinical discourse to describe individuals diagnosed with one or more of the following conditions: asthmatic bronchitis, chronic bronchitis, chronic obstructive bronchitis and emphysema” (5). Less common obstructive lung diseases include bronchiolitis obliterans and lymphangioleiomyomatosis. It is estimated that 14 million people in the United States have COPD, of which approximately 12.5 million have chronic bronchitis and 1.65 million have emphysema. COPD is the fourth leading cause of death in the United States (4). Asthma is also common, affecting approximately 11 million people (6). The prevalence of COPD, in particular asthma, is increasing (7,8). Given the strong association of COPD and cigarette smoking, this condition is largely preventable. Within any one patient features of emphysema, chronic bronchitis and asthma may overlap, and distinguishing between them may therefore be confounded. For example, some patients with asthma, particularly longstanding asthma, lack the reversibility of airflow obstruction that characterizes asthma, thereby mimicking COPD (7). Conversely, some patients with chronic bronchitis and emphysema have a significant component of bronchoreversibility, thereby mimicking asthma (9).
clinical discourse to describe individuals diagnosed with one or more of the following conditions: asthmatic bronchitis, chronic bronchitis, chronic obstructive bronchitis and emphysema” (5). Less common obstructive lung diseases include bronchiolitis obliterans and lymphangioleiomyomatosis. It is estimated that 14 million people in the United States have COPD, of which approximately 12.5 million have chronic bronchitis and 1.65 million have emphysema. COPD is the fourth leading cause of death in the United States (4). Asthma is also common, affecting approximately 11 million people (6). The prevalence of COPD, in particular asthma, is increasing (7,8). Given the strong association of COPD and cigarette smoking, this condition is largely preventable. Within any one patient features of emphysema, chronic bronchitis and asthma may overlap, and distinguishing between them may therefore be confounded. For example, some patients with asthma, particularly longstanding asthma, lack the reversibility of airflow obstruction that characterizes asthma, thereby mimicking COPD (7). Conversely, some patients with chronic bronchitis and emphysema have a significant component of bronchoreversibility, thereby mimicking asthma (9).
Table 15.1: Chronic Obstructive Pulmonary Diseases | |
|---|---|
|
Clinical History and Physical Examination
Cough, wheezing, and dyspnea are the common symptoms of obstructive lung disease and overlap between asthma and COPD. History can be useful to identify a specific obstructive lung disease, for example, a history of symptoms first appearing in childhood, a persistent cough after an upper respiratory tract infection, and/or a history of atopic disease or occupational exposure favor asthma (10,11). An episodic cough, perhaps brought on by exercise or allergens, also indicates an asthmatic component to obstructive lung disease. In contrast, patients with COPD generally have more constant and progressive symptoms; many patients with longstanding disease may be asymptomatic even in the face of severe disease (12). Age is an indicator of the type of obstructive lung disease. In one study, the mean age of patients with predominantly asthmatic bronchitis was 29.6 years versus 64.6 years for emphysematous COPD (2). Patients with α1-antitrypsin deficiency develop premature COPD, with a mean age of dyspnea onset at 40 years for smokers and 53 years for nonsmokers. Therefore, patients less than 50 years of age with moderate or severe chronic airflow obstruction, basilar emphysema, and a strong family history of obstructive disease, should be tested for α1-antitrypsin deficiency (4).
The most useful signs on physical examination of airflow obstruction are objective wheezing, barrel chest deformity, rhonchi, hyperresonance, subxiphoid apical impulses, and an objective measurement of prolonged expiration (11,13,14). Forced expiratory time may be useful to screen for obstructive lung disease; however, physicians are generally poor at estimating the severity of obstruction from physical examination. In one series, only 38% of physicians’ pretreatment estimates were accurate (15). The role of blood studies in the evaluation of obstructive lung disease is limited. An elevated IgE level and increased blood eosinophils may aid in identifying patients with asthmatic airflow obstruction. Patients with any history of asthma have a higher degree of eosinophilia and elevated IgE levels than patients without a history of asthma (3,16). However, patients with newly diagnosed chronic bronchitis may also have elevated IgE and eosinophil levels.
Pulmonary Function Testing
The most useful laboratory studies for the evaluation of airflow obstruction are pulmonary function tests. These tests are necessary for diagnosis, evaluating disease severity, and monitoring response to treatment (4,17). Spirometry is a simple screening test for airflow
obstruction, with spirometers widely available and simple to use. American Thoracic Society guidelines provide normal standards and the framework for the interpretation of results, with values adjusted for age and gender (Table 15.2) (18). The most valuable measurements include the FEV1, forced vital capacity (FVC), and peak inspiratory/expiratory flows measured from a maximal expiratory maneuver. A decrease in the FEV1/FVC ratio from the predicted range is diagnostic of airflow obstruction (18,19). A lower initial FEV1/FVC ratio in COPD may be associated with greater declines in FEV1 over time (20). Airflow obstruction severity is judged on FEV1 expressed as a percent of the predicted value (18). A graphic representation of peak expiratory and inspiratory flow versus lung volume, known as the flow-volume loop, should be examined to exclude potential upper airway obstruction (21).
obstruction, with spirometers widely available and simple to use. American Thoracic Society guidelines provide normal standards and the framework for the interpretation of results, with values adjusted for age and gender (Table 15.2) (18). The most valuable measurements include the FEV1, forced vital capacity (FVC), and peak inspiratory/expiratory flows measured from a maximal expiratory maneuver. A decrease in the FEV1/FVC ratio from the predicted range is diagnostic of airflow obstruction (18,19). A lower initial FEV1/FVC ratio in COPD may be associated with greater declines in FEV1 over time (20). Airflow obstruction severity is judged on FEV1 expressed as a percent of the predicted value (18). A graphic representation of peak expiratory and inspiratory flow versus lung volume, known as the flow-volume loop, should be examined to exclude potential upper airway obstruction (21).
Measurement of spirometric parameters before and after administration of a short-acting β agonist may aid in establishing bronchoreversibility. A rise in FEV1 of 12% with an absolute rise of at least 200 mL indicates bronchoreversibility (18). Complete reversibility of airflow obstruction makes the diagnosis of asthma likely and strongly argues against COPD (11). However, a subset of patients with asthma develop irreversible airflow obstruction (7,22,23,24). Such patients typically have a longer duration of asthma and more symptomatic disease than asthma patients with reversible obstruction (22). Furthermore, asthma patients with irreversible airflow obstruction may have baseline FEV1, FVC, and postbronchodilator FEV1 measurements indistinguishable from COPD patients; therefore, the lack of bronchodilator reversibility cannot be used with sufficient specificity to differentiate COPD from asthma (23). Up to 30% of patients with COPD have bronchoreversibility at spirometric testing (25,26). In a series of patients with asthma (n = 287) or COPD (n = 108), the mean increase in FEV1 from baseline after inhaled albuterol was higher in asthma patients (16.5% versus 10.6%); however, there was significant overlap (9). The best specificity for the diagnosis of asthma (84%) was defined at a threshold of a 20% or greater increase in FEV1 from baseline; however, sensitivity was poor. The postbronchodilator FEV1 has been shown to be the single best predictor of survival in patients with COPD, with a value below 30% of predicted associated with marked reduction in long-term survival (25). As a result, the postbronchodilator FEV1 has become instrumental in establishing an appropriate time for considering surgical therapy in patients with advanced COPD.
Spirometry can also be performed after the administration of an agent to induce bronchial hyperreactivity, such as methacholine (19,27). Patients with asthma have greater airway hyperresponsiveness than patients with COPD (27,28,29),30), although many patients with COPD also have airway hyperreactivity (27). In the Lung Health Study of almost 6,000 current smokers with borderline to moderate airflow obstruction, nonspecific airway hyperresponsiveness (defined as a drop of at least 20% in FEV1 after inhalation of no more than 25 mg/mL methacholine) was noted in 85.1% of women and 58.9% of men (31). Baseline airway obstruction, wheeze, cough with or without sputum production, and a past history of asthma or hay fever were associated with hyperresponsiveness.
Table 15.2: Categories of Obstructive Abnormality on Pulmonary Function Testing | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||
The measurement of lung diffusing capacity for carbon monoxide (DLCO) is a routine test in the evaluation of chronic airflow obstruction, particularly for more advanced disease. DLCO is a sensitive test for emphysema, a disease process associated with loss of alveolar surface area and pulmonary circulation (32). Decreased DLCO accompanying chronic airflow obstruction suggests at least a component of emphysema (33). The specificity of DLCO for obstructive lung disease is low, and it should be used together with clinical history and physical examination. In large epidemiologic studies, a normal DLCO is associated with asthma rather than COPD (34). DLCO may be also increased in patients with asthma (35,36). However, when asthma manifests with irreversible airway obstruction, DLCO may be low (23). In patients with emphysema, there is a strong correlation between low DLCO and greater severity of emphysema on CT (37). A decreased DLCO is associated with a more rapid decline in pulmonary function over time (20).
Peak expiratory flow is the maximal flow that can be achieved during maximal expiratory effort. This measurement has been widely accepted and advocated for the monitoring of patients with airflow obstruction, particularly asthma (17). Widespread availability of inexpensive, simple, reliable devices has made the routine measurement of peak expiratory flow rate possible (38). There is a close relationship between the FEV1 and peak expiratory flow rate; however, in general peak expiratory flow rate is consistently higher (38,39). Limitations of using peak expiratory flow rate include a reduction in peak expiratory flow rate both with obstructive lung disease and upper airway obstruction (21) and a lower sensitivity of peak expiratory flow rate than spirometry for detecting reversibility of airflow obstruction after bronchodilators or detecting bronchial response to challenge with occupational sensitizers (40,41).
Radiologic Studies
Posteroanterior and lateral chest radiographs are part of the initial evaluation of dyspnea. Many of the radiographic signs of obstructive lung disease lack specificity, such that chest radiographs are used predominantly to support a diagnosis of obstructive lung disease and not for primary diagnosis. Radiographic signs of obstructive lung disease also lack sensitivity and should not be used to exclude the diagnosis. The radiographic features of asthma, chronic bronchitis, and emphysema often overlap, similar to the overlap in clinical features. Although CT findings in the different forms of obstructive lung disease are well described and CT is the best tool to evaluate the severity of emphysema in vivo, CT has a limited role in the primary diagnosis of obstructive lung disease. In a small subset of dyspnea patients with an isolated reduction in diffusing capacity and otherwise normal pulmonary function tests and a normal chest radiograph, HRCT is useful for establishing the diagnosis of emphysema (42,43). When bronchiectasis is suspected, HRCT is the technique of choice for detecting, localizing, and characterizing bronchiectasis, having replaced bronchography. HRCT examinations can also be performed at end-expiration to add a functional component to the inspiratory HRCT technique. Expiratory CT may demonstrate air trapping in patients with small airway diseases such as asthma and bronchiolitis obliterans, often when inspiratory HRCT images are normal (44).
Asthma
Clinical Features and Definition
The diagnosis of asthma is based on clinical history and evidence of reversible airflow obstruction (17). Asthma is typically characterized by airway inflammation, airway hyperresponsiveness, and reversible airflow obstruction (7,45). Accurate diagnosis, monitoring of disease severity, and treatment response are considered the standard of care for asthma (17). Asthma is difficult to diagnose on purely clinical grounds (17,46). In one series of
60 adults with a physician diagnosis of asthma, 40% did not meet objective diagnostic criteria for asthma. Disease severity is often understated by both the patient and physician in the absence of spirometry or peak flow measurement (17).
60 adults with a physician diagnosis of asthma, 40% did not meet objective diagnostic criteria for asthma. Disease severity is often understated by both the patient and physician in the absence of spirometry or peak flow measurement (17).
Asthma is often thought of as a childhood illness, with many outgrowing the disease by adolescence if not adulthood. Asthma may persist into adulthood. Individuals diagnosed with asthma in childhood who ceased wheezing during adolescence have no difference in pulmonary function than normal control subjects, whereas those who continue to wheeze have abnormal pulmonary function. However, 60% of patients who stopped wheezing have evidence of bronchial hyperresponsiveness with histamine challenge (47). The outcome of childhood asthma is predominantly related to the initial level of bronchial obstruction and airway responsiveness. Lack of asthma at follow-up is associated with younger age and less severe airway obstruction when first tested, whereas the absence of bronchial hyperreactivity is associated with younger age, higher FEV1, and shorter untreated period (48). Milder disease and early intervention are important components to asthma outcome. Tobacco smoke has a deleterious effect on asthma. Asthmatics exposed to greater than 3 hours of smoke per week have greater severity of asthma scores, worse asthma-specific quality of life scores, and decreased SF-36 health status questionnaire scores and increased odds for emergency room visits, urgent doctor visits, and hospitalizations compared with non–smoke-exposed asthma control subjects (49).
The development of asthma is multifactorial and includes family history of asthma and current or former smoking (50). The odds of having a child with asthma are three times greater in a family in which one parent has asthma and six times more likely when both parents have asthma and are greater if the mother has asthma than if the father has asthma (51). In one series of 265 first-degree offspring of asthma patients, 18% of the offspring had definite asthma and 8% had probable asthma (52).
Pathophysiology
Inflammation is now recognized to have a large role in asthma (7). Even in patients with mild asthma there is collagen deposition beneath the epithelial basement membrane and extensive inflammation in bronchial biopsy specimens (53). There are increased numbers of inflammatory cells and eosinophils in the airway epithelium of asthmatics, and these cells are associated with increased bronchial reactivity. Greater subepithelial thickening correlates with lower FEV1 and greater peak flow variability, suggesting that the clinical severity of asthma is associated with both the severity of inflammation and also the degree of airway remodeling (54). Patients with COPD who demonstrate corticosteroid reversibility may have features of asthma and inflammation, with COPD responders having a larger number of eosinophils and higher levels of eosinophilic cationic protein in bronchoalveolar lavage fluid and thicker reticular basement membrane than COPD nonresponders (55).
Airway inflammation plays an important role in asthma.
Chest Radiography and Computed Tomography
The chest radiographic features of asthma include pulmonary hyperinflation with increased lung lucency and mild bronchial wall thickening (Fig. 15.1). However, the chest radiograph is often normal, particularly in the absence of acute asthma symptoms. Mild pulmonary artery enlargement may occur due to transient pulmonary hypertension (56). During acute exacerbation of asthma, atelectasis, mucous plugging, spontaneous pneumomediastinum, or pneumothorax may develop. The latter occurs due to air trapping with a ball–valve phenomenon, allowing air into the lungs with inspiration but little if any exit of air on expiration. HRCT examinations are frequently abnormal in asthma patients with normal chest radiographs. In one series 71.9% of asthmatics had an abnormal HRCT, whereas only 37.8% of these patients had an abnormal chest radiograph (57). HRCTs may be normal, particularly in mild asthma.
Radiographs are often normal in asthma patients.
HRCT may show bronchial wall thickening or air trapping in asthma not evident on chest radiographs.
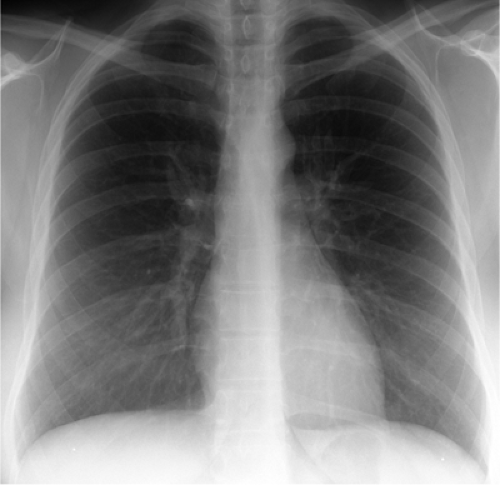 Figure 15.1 Posteroanterior chest radiograph of a patient with asthma demonstrates pulmonary hyperinflation and peribronchial cuffing. |
Bronchial dilatation occurs in 28% to 36% of asthma patients and bronchial wall thickening in 82% to 92% of asthma patients (58,59). Reversible findings on HRCT include mucoid impaction and lobar collapse, present in 10% to 20% of asthmatic patients (57,58). In longstanding asthma, bronchial dilatation and bronchial wall thickening on HRCT are often irreversible (23,57,58,60). Bronchial wall thickening and bronchial dilatation are more common and more severe in asthmatic patients with moderate to severe airflow obstruction and in patients with a prolonged history of asthma than in patients with mild obstruction or normal airflow (61,62,63,64).
Air trapping is commonly demonstrated on expiratory HRCT and may precede the development of airway dilatation and thickening (Fig. 15.2) (60). Patients with nonallergic asthma have more extensive airway remodeling on CT than patients with allergic asthma, with a higher frequency of bronchial dilatation, bronchial recruitment, and emphysema (63). In contrast, patients with chronic stable asthma develop a reduction in
lung attenuation on HRCT that is not due to emphysema. In nonsmoking asthmatic patients, emphysema is not a feature of asthma on HRCT (65,66). This reduction in lung attenuation may represent nondestructive hyperinflation.
lung attenuation on HRCT that is not due to emphysema. In nonsmoking asthmatic patients, emphysema is not a feature of asthma on HRCT (65,66). This reduction in lung attenuation may represent nondestructive hyperinflation.
Some lung findings on HRCT in mild asthmatics can be provoked and are reversible. After bronchial provocation with methacholine chloride, a reduction in lung attenuation and reduction in the cross-sectional area of small airways (less than 5 mm2) occurs compared with baseline, accompanied by a 10% to 26% decrease in FEV1. After reversal with albuterol, these findings return to normal (67). After methacholine inhalation, the internal airway lumen diameter has been shown to decrease 17% from baseline, increasing to 18% above baseline after albuterol inhalation (68). Methacholine-induced bronchial constriction occurs in bronchi of all sizes but is most severe in the small bronchi 2 to 4 mm in diameter (69). Although in normal patients a decrease in bronchial wall thickness accompanies bronchoconstriction, bronchial wall thickness does not decrease in asthmatic patients as measured on HRCT (69).
Chronic Bronchitis
Chronic bronchitis is diagnosed clinically by the presence of chronic productive cough for 3 months in each of 2 successive years in a patient in whom other causes of chronic cough have been excluded (4). Compared with asthma and emphysema, the radiographic features of chronic bronchitis are poorly described. Findings at chest radiography include pulmonary hyperinflation and thickened bronchial walls, resulting in peribronchial thickening or cuffing, and increased “markings” due to superimposition of the thickened small bronchi and bronchiole walls (70). There is little information on the HRCT findings in chronic bronchitis. The most common HRCT finding is bronchial wall thickening, a nonspecific finding (71). In one series of 45 patients with air trapping on expiratory HRCT, 4 patients with chronic bronchitis were reported (44). All four patients had air trapping on expiratory HRCT, and one patient had a normal inspiratory HRCT. In the remaining three patients, the inspiratory HRCT demonstrated bronchial wall thickening, a tree-in-bud appearance secondary to mucoid impaction, and ground glass opacity presumed to be secondary to concomitant infection.
Emphysema
According to the American Thoracic Society, emphysema is defined as “a condition of the lung characterized by abnormal, permanent enlargement of the air spaces distal to the terminal bronchiole, accompanied by destruction of their walls” and without obvious fibrosis (4). Emphysema occurs due to an imbalance in the proteolytic activity in the lungs, resulting in destruction of alveolar tissue. This may be seen with an overabundance of proteolytic enzymes, a lack of antiproteases, or a combination of both. In smoking-related centrilobular emphysema there is excess protease, whereas in α1-antitrypsin deficiency there is a deficiency of α1-antiproteinase.
The pathologic classification of emphysema is based on the secondary pulmonary lobule. The four major categories of emphysema, as listed in Table 15.3, are centrilobular (centriacinar),
panacinar (panlobular), paraseptal, and paracicatricial (Fig. 15.3) (72). Table 15.4 summarizes the differences between centrilobular and panlobular emphysema. Centrilobular emphysema is the most common form of emphysema and is usually secondary to cigarette smoking. The destruction of alveolar walls begins in the central portion of the secondary pulmonary lobule (Fig. 15.4) and is heterogeneous, affecting adjacent lobules with varying degrees of severity; it is usually most severe in the upper lobes (Fig. 15.5). The relatively greater ventilation-perfusion ratio in the upper portion of the lungs compared with the lung bases favors greater deposition of the particulate matter from cigarette smoke in the upper lungs. Activated macrophages release the proteolytic enzyme, elastase; free radicals and oxidants in cigarette smoke inactive normally protective antiproteases, leading to greater destruction of the upper lobes than the lower lobes (72).
panacinar (panlobular), paraseptal, and paracicatricial (Fig. 15.3) (72). Table 15.4 summarizes the differences between centrilobular and panlobular emphysema. Centrilobular emphysema is the most common form of emphysema and is usually secondary to cigarette smoking. The destruction of alveolar walls begins in the central portion of the secondary pulmonary lobule (Fig. 15.4) and is heterogeneous, affecting adjacent lobules with varying degrees of severity; it is usually most severe in the upper lobes (Fig. 15.5). The relatively greater ventilation-perfusion ratio in the upper portion of the lungs compared with the lung bases favors greater deposition of the particulate matter from cigarette smoke in the upper lungs. Activated macrophages release the proteolytic enzyme, elastase; free radicals and oxidants in cigarette smoke inactive normally protective antiproteases, leading to greater destruction of the upper lobes than the lower lobes (72).
Table 15.3: Major Categories of Emphysema | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
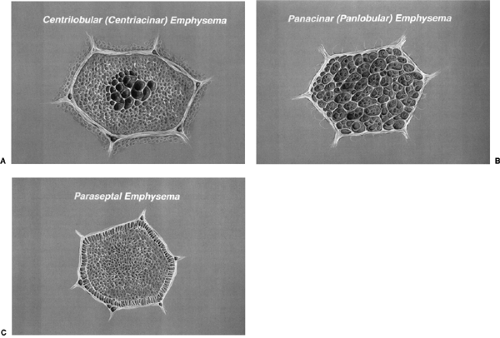 Figure 15.3 Illustration of centrilobular, panacinar, and paraseptal emphysema at the level of the secondary pulmonary lobule. A. Enlargement of the central airspaces of a secondary pulmonary lobule in centrilobular emphysema. B. Enlargement of airspaces uniformly throughout the secondary pulmonary lobule in panlobular emphysema. C. Enlargement of airspaces along the periphery of the lobule adjacent to the interlobular septa in paraseptal emphysema. (From
Get Clinical Tree app for offline access
Kazerooni EA, Whyte RI, Flint A, et al. Imaging of emphysema and lung volume reduction surgery. RadioGraphics 1997;17:1023–1036
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree


|