8
Normal Anatomy and Flow During the Complete Examination
Epiaortic Imaging
 Introduction
Introduction
Epiaortic ultrasound (EAU) is an imaging modality whereby a handheld transducer is placed directly upon a surgically exposed aorta. EAU provides high-quality sonographic data depicting aortic anatomy and pathology. The use of ultrasound imaging directly applied to the surgical field predates the introduction of intraoperative transesophageal echocardiography (TEE).1,2 Epicardial ultrasound was used in the 1980s at The Mount Sinai Medical Center and at Columbia University College of Physicians and Surgeons in New York City to assess left ventricular function, 3 myocardial perfusion, 4 the presence of intracardiac air, 5 and mitral valve function after mitral valve repair. 6 In addition to epicardial ultrasound, EAU is a useful tool in the echocardiographer’s armamentarium to address clinical situations such as evaluating a diseased aorta in the face of aortic manipulation or instrumentation.
The most recent guidelines by the American Society of Echocardiography (ASE) and the Society of Cardiovascular Anesthesiologists (SCA) recommend five standard views. 7 Its ease of use, minimal time to perform, negligible complication rate, and high accuracy has made EAU an appealing tool. The guidelines recommend its use in patients at high risk for embolic events, but also speculated on more widespread use of EAU, given its favorable risk/benefit ratio.
 Indications
Indications
The initial impetus for the use of EAU stemmed from attempts to reduce the rate of perioperative stroke during cardiac surgery. The 2008 ASE/SCA guidelines for comprehensive intraoperative ultrasonographic examination recommend the use of EAU in patients with increased risk for embolic stroke, history of cerebrovascular disease, peripheral vascular disease, and patients in whom other imaging modalities demonstrate the presence of aortic atherosclerotic disease. 7 Risk factors for stroke in cardiac surgery include advanced age, female gender, proximal aortic atherosclerosis, calcified aorta, history of cerebrovascular disease, peripheral vascular disease, diabetes, hypertension, prior cardiac surgery, preoperative infection (including endocarditis), urgent surgery, greater than 2-hour cardiopulmonary bypass (CPB) time, intraoperative hemofiltration, and transfusion. EAU can identify diseased aortic segments containing atherosclerotic plaque, calcification, or thrombus that are at high risk for distal embolization. 8 Once these areas are identified, the surgical approach to the aorta may be altered or aborted to reduce this risk. 9 In addition to possible alterations in surgical strategy, the examination can provide information for stratification of a patient’s risk of complication from distal embolization.
 Stroke
Stroke
Stroke (cerebrovascular accident) is a potentially devastating complication of an otherwise successful cardiac operation. Although there are many types of cerebral injury after CPB, such as transient ischemic attack, delirium, and cognitive dysfunction, this chapter will focus on stroke. Stroke is defined by the World Health Organization as a “neurological deficit of cerebrovascular cause that persists beyond 24 hours or is interrupted by death within 24 hours.” Neurologic complications are the second most common causes of morbidity and mortality after cardiac surgery, second only to heart failure. Stroke can lead to delayed discharge from a long-term care facility, increased hospital stay, and increased mortality.10,11 The incidence of perioperative stroke varies in the literature, based on study design and the population examined.12–14 The stroke rate clearly increases with age, cardiovascular comorbidities, CPB time, and the complexity of the operation. In a published review of the Society of Thoracic Surgeons (STS) database from 2002 to 2006 examining over 700,000 CABG operations and over 100,000 mitral valve surgeries, the stroke rates were most common in combined mitral valve and coronary artery bypass grafting (CABG) and least common in isolated CABG ( Table 8-1).15–17
TABLE 8-1
Society of Thoracic Surgeons Stroke Rates for Coronary Artery Bypass Graft and Valve Surgery (2002-2006) ∗
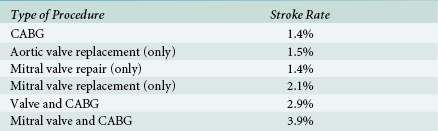
∗Stroke rates as described by 2002-2006 Society of Thoracic Surgeons database. Most frequent cohort of patients to suffer stroke is the group that received mitral valve and coronary artery bypass graft (CABG) surgery. Smallest stroke risk was in the isolated CABG surgery group.
Data from Shahian DM, et al. The Society of Thoracic Surgeons 2008 cardiac surgery risk models: part 1—coronary artery bypass grafting surgery. Ann Thorac Surg. 2009;88:S2; O’Brien SM, et al. The Society of Thoracic Surgeons 2008 cardiac surgery risk models: part 2—isolated valve surgery. Ann Thorac Surg. 2009;88:S23; Shahian DM, et al. The Society of Thoracic Surgeons 2008 cardiac surgery risk models: part 3—valve plus coronary artery bypass graft surgery. Ann Thorac Surg. 2009;88:S43.
Additional risk factors are similar to those encountered for stroke in the general population. These include prior stroke, diabetes, female gender, renal failure, hypertension, atrial fibrillation, peripheral vascular disease, and others.18–22 Most relevant for our discussion is the risk of stroke conferred by severe atherosclerotic disease of the thoracic aorta, as screened by ultrasound as discussed earlier. Although there has been controversy regarding the importance of aortic atherosclerosis as a risk factor for primary strokes in the general population,23–34 it has now been strongly established in the cardiac surgery population that aortic plaque correlates with postoperative stroke incidence.9,35–42 The more extensive and complex the plaque burden, the higher the risk of postoperative stroke.
Possible mechanisms of stroke include arterial-to-arterial embolization of plaque or thrombus, embolization from intracardiac sources, or paradoxical embolization from venous to arterial circulations across intra- or extracardiac shunts.43–48 Other etiologic pathways include in situ arterial thrombosis that leads to critical obstruction of brain perfusion, as well as a low-flow state causing ischemic injury due to hypotension and reduced cardiac output. Air entrainment from open cardiovascular structures has been implicated in both stroke and more subtle neurologic impairments after cardiac surgery. 49 Stroke may also be caused by cerebral hemorrhage, decreased cerebral venous drainage, and as a sequela of prolonged seizure activity. Based on radiographic studies, it appears that embolization to the cerebral arterial system is the most common cause of stroke after cardiac surgery.50,51
Three common types of matter that embolize to cause neurologic injury are cholesterol-laden atheromas, particles of embolized thrombus, and entrained air. It is likely that the larger the embolic particle and the more frequent the occurrence of embolic events, the greater the risk and severity of neurologic injury.52,53 It is also thought that low-flow states augment the ischemic injury from embolized particles by failing to “wash out” the cerebral circulation of these obstructing particles.54–56
During cardiac surgery, the aorta is manipulated in several ways that may lead to plaque disruption and embolization. These include aortic clamp placement and removal, insertion of the aortic cannula, the antegrade cardioplegia vent, the proximal anastomosis of coronary grafts, as well as the aortotomy itself. Flow from the aortic cannula while on CPB may cause a “sandblasting effect” that may disrupt plaque downstream from the cannula.57,58
Several methods are used to detect and characterize the patient’s atheromatous burden within the ascending aorta. Preoperatively, plaque and calcification may be detected by means of radiologic scanning with computed tomography (CT) or magnetic resonance imaging (MRI). Intraoperatively, three methods are currently employed: direct surgical palpation of the aorta to feel for the hardness of calcification, TEE, and EAU. Several studies clearly demonstrate the superiority of TEE and EAU over surgical palpation. Surgical palpation is much less sensitive than ultrasound, considerably underestimating the atherosclerotic burden; EAU has the greatest sensitivity.59–67 In one study, Linden et al. examined 921 consecutive cardiac surgery patients. EAU revealed that 26% had plaque on the ascending aorta measuring more than 5 mm thick, but surgical palpation only detected 40% of these lesions. 30 Calcified areas the surgeon is able to feel may be stable plaques with a lower risk for embolization than the soft atheromas that may only be detectable by ultrasound. 68
Although the TEE probe may already be in place and its use does not interrupt the surgical flow, EAU offers several advantages over TEE for characterizing aortic plaque. Most surgical manipulation occurs in the region of the ascending aorta considered to be the “blind spot” of TEE. This is where the trachea and left mainstem bronchus interpose between the esophagus and aorta, causing disruption of ultrasound transmission. Thus, although parts of the ascending aorta can be visualized by TEE, 69 Konstadt et al. have shown that TEE is frequently unable to image the region of greatest interest of the ascending aorta. 70 Since EAU is placed closer to the area of interest, enabling higher transducer frequencies to be used, higher-quality images with fewer artifacts are possible.
Well-designed trials demonstrating a morbidity or mortality benefit to EAU are lacking, but an accumulating body of data indicates that in high-risk patients the use of EAU alters surgical management and that these changes may be beneficial.56,71–78 Some examples of modifications of surgical technique are off-pump (as opposed to on-pump) CABG, “no-touch” techniques, and alterations in the site of aortic manipulation and instrumentation (e.g., axillary cannulation).79–82 More radical surgical approaches may include aortic endarterectomy or aortic arch replacement, although these approaches may produce worse outcomes. 83
 Probes and Technique
Probes and Technique
The linear probe scans both the anterior aortic wall (near field) and the posterior aortic wall (far field) without the need for a transducer standoff. One drawback is that linear probes tend to have a large “footprint,” which refers to the large surface area of the probe itself. Thus, a linear probe may be difficult to maneuver in a small surgical field. Second, the entire left-to-right dimensions of the aorta may not fit in a single ultrasonographic window, creating a “tunneled” view, and the transducer may have to be repositioned rightward and leftward to acquire the entire cross-sectional picture of the aorta ( Fig. 8-1).
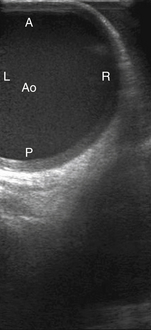
Figure 8-1 Epiaortic ultrasound image of tubular ascending aorta (Ao) using linear probe. A, Anterior; L, left lateral wall; P, posterior; R, right lateral wall.
The wedge-shaped images of the phased and matrix array transducers enable simultaneous imaging of the right and left aortic walls, and they also tend to have a smaller footprint, enabling greater maneuverability within the surgical field. The drawback of phased or matrix array transducers is the need for a standoff. Because of the wedge shape of the image, if the transducer is placed directly on the aorta, the anterior aortic wall (near field) will not be completely imaged; only a small section will be displayed while the rest will be outside the sector. To capture the near field in its entirety, the transducer is held at some distance away from the aorta (the standoff) ( Fig. 8-2). This requires addition of a medium that conducts ultrasound waves. Air cannot be used because it is a poor acoustic conductor. The standoff may be provided by a gel that can either be purchased as a pad or as gel already incorporated into the sterile cover wrapping surrounding the transducer tip. Another simple approach is to fill the sterile condom sheath with a column of saline, enabling the transducer to be held away from the aorta while transmitting ultrasound waves though the saline to the aorta. At our institution, we place sterile gel within the sterile sheath, fill the pericardial well with warm sterile saline, and hold the transducer immersed in the saline at a distance from the aortic wall so that the anterior wall of the aorta can be visualized in its entirety.
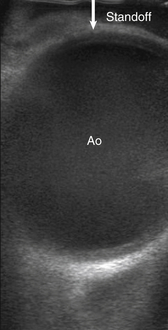
Figure 8-2 Epiaortic ultrasound of ascending aorta (Ao) with “standoff,” the distance held off image being interrogated by probe.
It is of great importance to maintain sterility when introducing the handheld transducer onto the surgical field. The probe is passed from the nonsterile area into a sterile condom sheath on the surgical field. Some institutions place a double sheath, exercising extra precaution. At our institution, we disinfect the transducers between cases and pass these probes into a sterile sheath from the field. It is important to note that different manufacturers recommend specific disinfection techniques for their probes; one must check with the manufacturer to identify the appropriate cleaning technique (see Chapter 29).
 Imaging Planes
Imaging Planes
The ascending aorta and arch should be systematically scanned throughout their entirety, paying particular attention to the sites of proposed aortic manipulation. Several papers have proposed standardized schemes for aortic scanning.7,84,85 To complete a comprehensive EAU examination from the sinotubular junction to the innominate artery and aortic arch, a minimum of five views are recommended. Usually, TEE allows for visualization of the proximal ascending aorta and distal aortic arch. 86
The ascending aorta should be evaluated in short and long axis in each of the proximal, mid-, and distal segments. In short axis with the ultrasound probe perpendicular to the aorta, the aorta should be measured from the near-field inner edge to the far-field inner edge of the aorta in each segment. Manipulation of the probe in perpendicular orientation as the probe is moved from proximal to distal allows for evaluation of all three portions of the ascending aorta. The proximal ascending aorta is demarcated by the beginning of the sinotubular junction, where it is common to see the aortic valve and right pulmonary artery. The mid–ascending aorta is defined by the part of the aorta that is juxtaposed with the right pulmonary artery ( Fig. 8-3). The distal ascending aorta is from the distal right pulmonary artery to the innominate artery. Movement farther distally will allow for examination of the proximal aortic arch, which is necessary if not clearly seen on TEE ( Fig. 8-4).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


