Fig. 12.1
The paracorporeal pulsatile left ventricular assist device used by Dr. Michael E. DeBakey in the 1960s (image property of Texas Heart Institute)
Throughout the 1960s and 1970s, several programs were initiated by the National Heart Institute (which later became the National Heart, Lung, and Blood Institute or NHLBI) to develop cardiac assist devices that could assist or replace the failing heart [7]. These programs produced several of the first-generation implantable pulsatile LVADs, including the HeartMate XVE (Thoratec Corporation, Pleasanton, CA), the Novacor Left Ventricular Assist System (World Heart, Inc., Oakland, CA), and the LionHeart Left Ventricular Assist System (Arrow International, Reading, PA). Altogether, these devices were implanted as bridges to transplantation or as destination therapy in several thousand patients worldwide who were not transplant candidates [8–10]. Currently, the Novacor and LionHeart devices are no longer being used clinically, although the HeartMate XVE is still available and used at many institutions.
In the mid-1980s, a miniature axial-flow blood pump (Fig. 12.2), the Hemopump―developed by Richard Wampler, MD, and the Nimbus Corporation [11]―underwent preclinical testing and first clinical use at the Texas Heart Institute in Houston [12]. This experience confirmed that a miniature rotary blood pump could unload the compromised left ventricle and provide adequate systemic support while improving the potential for myocardial recovery. In 1994, NHLBI initiated the innovative ventricular assist systems program to encourage the development of a totally implantable ventricular assist system [13]. This program resulted in the next generation of smaller, implantable continuous-flow blood pumps [14–17].


Fig. 12.2
The Hemopump miniature axial-flow blood pump is about the size of the eraser on an ordinary pencil. The successful clinical use of this device encouraged further development of implantable rotary blood pumps (image property of Texas Heart Institute)
Today, numerous rotary blood pumps are undergoing clinical trials designed to test their safety and effectiveness so that they may be used as bridges to transplantation, bridges to recovery, or destination therapy. Rotary pumps in current clinical use can be classified as to whether they have axial or centrifugal flow, have magnetically suspended or attached bearings, or are suitable for short- or long-term use. Axial-flow and centrifugal pumps typically have a central rotor containing permanent magnets. Electrically controlled coils, embedded in the housing of the blood pump, couple with magnets located in the impellers, thereby controlling rotational speed. Centrifugal pumps typically contain rotors shaped to accelerate blood flow towards the outer wall of the pump. In contrast, axial-flow pumps typically contain rotors that are more cylindrical. Helical blades on these cylindrical rotors cause blood flow to accelerate along the spinning axis of the rotor.
Another feature of continuous-flow pumps is the method used to suspend the rotor or impeller. Early continuous-flow pumps used solid fixed bearings, which are vulnerable to friction-related bearing wear, do not allow for washout of tight gaps, and sometimes allow thrombus to build up. Today, many continuous-flow pumps utilize electromagnetic and hydrodynamic suspension, which can virtually eliminate pump wear and also reduce damage to blood cells. Compared to earlier LVADs, these devices are much smaller and simpler, having only one moving part; as a result, they are less susceptible to infection and failure. They require a less invasive implant procedure and fit a wider size range of patients. Today’s continuous-flow pumps are not only more efficient than previous models but are also much more comfortable for patients, allowing them to return to a relatively normal lifestyle.
This chapter focuses on the current use of continuous-flow pumps for both temporary and long-term support of patients with acute and chronic heart failure.
Short-Term Support
Continuous-flow pumps designed for temporary use may be appropriate in several clinical settings involving cardiogenic shock (e.g., acute myocardial infarction or postcardiotomy heart failure) and to provide cardiac support during coronary revascularization procedures. Although these devices may be inserted by cardiovascular surgeons in the operating room, they are usually and most effectively applied by cardiologists in the catheterization laboratory. Short-term pumps may also be used for bridging to a long-term cardiac assist device. The systems described in this section have been approved by the United States Food and Drug Administration (FDA) and been used for cardiac support in thousands of cases, for either left ventricular, right ventricular, or biventricular support.
Impella
The Impella System (Abiomed, Inc., Danvers, MA) consists of a small axial-flow blood pump that is similar to the earlier Hemopump. Three Impella models are used for left-sided cardiac support: the Impella LP 2.5, Impella LP 5.0, and Impella LD. All three models are catheter-mounted axial-flow pumps and have a distal cannula that is placed across the aortic valve in retrograde fashion to directly unload the left ventricle. The pump and pump outlet are located in the ascending aorta, into which blood is ejected for systemic support. The Impella RD is used for right-sided cardiac assistance and is connected to the right atrium and pulmonary artery. This device has CE mark approval and is undergoing clinical trials in the United States. Currently, the Impella 2.0 and 5.0 have both FDA and CE mark approvals.
The Impella pumps are connected to an external drive unit by a 2.8-mm flexible drive cable and a purge system (using a heparinized 40 % glucose solution), which continuously flushes the pump and motor housing.
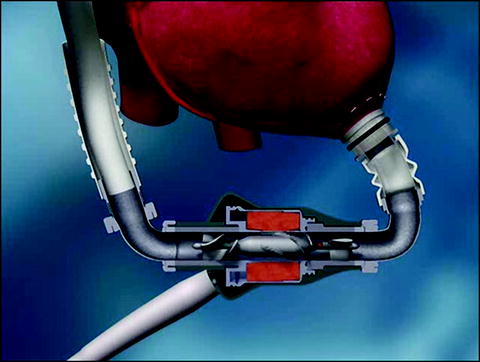
The Impella LP 2.5 consists of a 4-mm (12F) microaxial blood pump mounted on a 9F pigtail catheter (Fig. 12.3). It is inserted percutaneously, with the aid of fluoroscopic guidance, in either the catheterization laboratory or the operating room. The device is inserted through the femoral artery via a 13F sheath over a guidewire. The system can provide up to 2.5 L/min of support against a normal physiologic afterload.
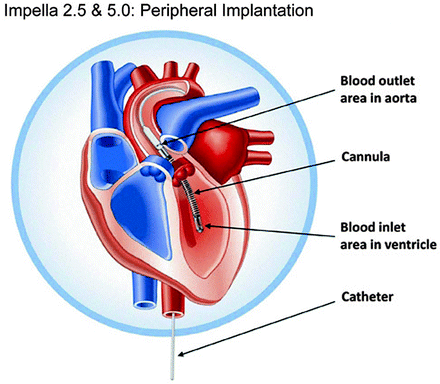

Fig. 12.3
The Impella 2.5 and 5.0 axial-flow pumps (Abiomed, Inc., Danvers, MA) are inserted via the femoral artery and advanced across the aortic valve for short-term cardiac support. The two pumps are exactly alike, except that the 5.0 model is larger
The Impella LP 5.0―a larger version of the Impella LP 2.5 device―consists of a 6.4-mm microaxial pump and a 7.3-mm-diameter (21F) cannula. The device is inserted into the femoral artery via a prosthetic graft and is advanced into the left ventricle with the aid of fluoroscopic guidance. It can provide up to 5.0 L/min of blood flow.
The Impella LD is the same as the Impella LP 5.0 but has a shorter (55-mm) left ventricular cannula (Fig. 12.4). The device is surgically implanted directly into the left ventricle via the ascending aorta.
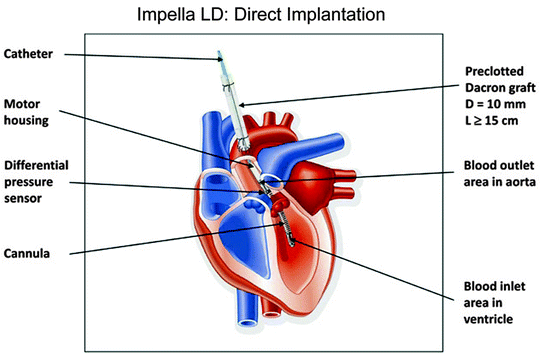

Fig. 12.4
The Impella LD (Abiomed, Inc., Danvers, MA) has a shorter cannula than the Impella 2.5 or 5.0. The LD is inserted surgically via the ascending aorta and advanced across the aortic valve
The Impella RD is used for right-sided support alone and can only be inserted surgically. This small microaxial-flow blood pump has a short caged inlet that is inserted directly into the right atrium and a ringed outlet graft that is anastomosed to the pulmonary artery. It can deliver blood flows of up to 5 L/min.
TandemHeart
The TandemHeart (Cardiac Assist, Inc., Pittsburgh, PA) is a percutaneously inserted left ventricular assist system for temporary use during high-risk percutaneous coronary interventions (PCIs) or off-pump coronary bypass; it is also used for bridging to transplantation and for treating acute myocardial infarction and postcardiotomy shock. The two-stage catheter system dilates the puncture site in the atrial septum and provides transseptal access by means of a transseptal cannula that drains blood from the left atrium (Fig. 12.5). An extracorporeal centrifugal blood pump returns blood to an arterial femoral cannula, which is positioned at the level of the femoral bifurcation.
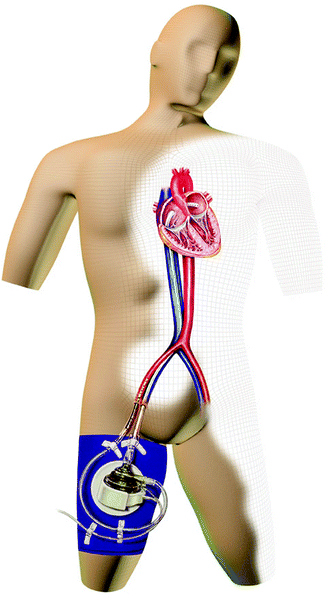

Fig. 12.5
The cannulas of the TandemHeart (CardiacAssist, Inc., Pittsburgh, PA) are inserted via the femoral artery and vein. The inflow cannula is placed within the left atrium by means of a transseptal technique
Use of this technique to support the failing left ventricle dates back to the 1950s, when Dennis and colleagues [5] used a transseptal approach to cannulate the left atrium, bypass the left ventricle, and return blood to the femoral artery with a roller pump. Seven of their eight patients survived for a short period, but there were no long-term survivors because revascularization procedures had not yet been developed.
The FDA-approved TandemHeart percutaneous ventricular assist device represents a clinically meaningful application of Dennis’s concept [18–21]. The external centrifugal pump weighs 227 g, operates at 3,000–7,500 rpm, and supplies up to 4 L/min of continuous flow [22]. Pump inflow is achieved through a 21F cannula that is placed in the left atrium through an atrial transseptal puncture via the femoral vein. The cannula draws oxygenated blood from the left atrium into the external centrifugal pump. A 15F or 17F outflow cannula is placed in the femoral artery. The pump controller, which rotates and controls the impeller of the centrifugal pump, as well as an anticoagulant infusion line, protects the hydrodynamic bearing by cooling it and providing an anticoagulation agent.
CentriMag
The CentriMag ventricular assist system (Levitronix LLC, Waltham, MA) is used for short-term left, right, or biventricular support (Fig. 12.6). The extracorporeal centrifugal blood pump contains a rotor that is magnetically levitated and spins friction free. The rotor is encased in a polycarbonate housing. The inlet to the blood pump is concentric with the axis of the rotor, and the pump outlet is perpendicular to the inlet. Blood enters via the inlet and contacts the spinning rotor; energy in the form of pressure and velocity is then transferred from the rotor to the blood, which exits the outlet port at flows of up to 10 L/min. The blood pump is attached to a motor which, when magnetically coupled, powers the pump. A cable connects the motor to a console that controls pump speed and monitors pump function.


Fig. 12.6
The CentriMag Ventricular Assist System (Levitronix LLC, Waltham, MA) (image property of Texas Heart Institute)
The cannulas are inserted into either the right or left atrium (for venous drainage) or into the pulmonary artery or aorta, depending on the type of support required. The cannulas are attached to standard 3/8-inch tubing, which is connected to the pump’s inflow and outflow ports. For continuous pump-flow monitoring, an ultrasonic flow probe is connected to the outside of the tubing and is directly connected to the console.
A cannulation technique that allows the chest to be completely closed and that avoids reoperation has been revived from the 1960s. By placing the cannula through a graft [23, 24], the surgeon can avoid reopening the chest when weaning is deemed adequate. Instead, the inlet cannula is removed from the inside of the graft, and the graft is oversewn. The outflow graft may be similarly oversewn. This unique method allows quick explantation.
The FDA-approved CentriMag pump is widely used in both Europe and the United States for the short-term support of patients with potentially recoverable heart failure. It is valuable for stabilizing the condition of patients with multiorgan system failure and an uncertain neurologic status. It is also used for supporting patients after an acute myocardial infarction, for bridging to recovery, for postcardiotomy shock, and for short-term right ventricular assistance in combination with implantable LVADs.
Long-Term Support
Experience gained with implantable pulsatile blood pumps for long-term support helped pave the way for the widespread use of continuous-flow rotary blood pumps for this purpose. Typically, these pumps are smaller and require less power than their pulsatile predecessors. Although continuous-flow devices have no flexing or moving diaphragms, no membranes, and no valves to ensure unidirectional flow, they generally do necessitate chronic anticoagulation therapy (i.e., warfarin). Percutaneous drivelines are also smaller than those of first-generation pumps, and improved management techniques have decreased the incidence of infection. Nevertheless, complications (e.g., percutaneous driveline site infections or external pump cable fractures) still do exist.
HeartMate II
The HeartMate II LVAD (Thoratec Corporation, Pleasanton, CA) consists of an implantable axial-flow blood pump, a controller module, an alternating-current power-based unit, and external batteries. The small blood pump measures 4 cm in diameter and 6 cm in length; it weighs 375 g and has one moving part, a high-speed impeller that spins on inlet and outlet ball-and-cup bearings. The impeller is contained within the pump housing (Fig. 12.7), which surrounds a brushless direct-current motor that creates a spinning magnetic field to activate the impeller. Rotational speeds range from 6,000 to 15,000 rpm, and the device can provide up to 10 L/min of continuous output. The sintered titanium inflow cannula is inserted into the left ventricle via a sewing ring, and blood is returned via a 12-mm outflow graft anastomosed to the ascending aorta (Fig. 12.8). Power is delivered via a percutaneous lead that exits the right upper portion of the abdomen; the lead is connected to external controllers and either an AC-power-based unit or wearable portable batteries. This system offers patients a greatly improved quality of life.