Baseline
1 year
1.5 years
2 years
MR grade
2.54 ± 0.81 (N = 70)
0.52 ± 0.66* (N = 46)
0.35 ± 0.63* (N = 33)
0.48 ± 0.62* (N = 26)
LV EF
37.9 ± 11.7 (N = 67)
47.0 ± 12.5** (N = 46)
46.5 ± 11.8** (N = 33)
47.0 ± 12.9*** (N = 25)
LVEDD (cm)
5.83 ± 0.68 (N = 60)
5.34 ± 0.86** (N = 38)
5.55 ± 0.80 (N = 26)
5.16 ± 0.75*** (N = 17)
LVESD (cm)
4.66 ± 0.89 (N = 57)
3.94 ± 1.08** (N = 35)
4.26 ± 1.03 (N = 23)
3.96 ± 0.95*** (N = 17)
From these data, we can conclude that CABG with reduction annuloplasty for FIMR predictably reduces MR and relieves symptoms in patients without excessive preexisting ventricular distortion. This operative strategy for the treatment of moderate to severe MR is associated with improved indices of ventricular geometry, improved NYHA functional class, and excellent freedom from recurrent mitral insufficiency. While long-term prognosis and outcomes remain uncertain, this dataset delineates the midterm benefits of such an approach.
Future Approaches in the Treatment of Functional Ischemic MR
Novel Clinical Research
One characteristic of functional MR is the presence of “normal” leaflet structure in the setting of ventricular remodeling which distorts the subvalvular apparatus and impairs valve function. A novel approach to treating this FMR is offered by the Coapsys device (Myocor, Inc., Maple Grove, MI), a ventricular shape change device that can be placed without the need for cardiopulmonary bypass to reduce FMR. This device consists of two pads which are connected by a transventricular “chordal” suture. After echocardiographically assisted placement across the left ventricle, the device is tightened to compress the mitral annulus, thereby reducing FMR and positively reshaping the ventricle [35]. An FDA-monitored investigational device trial was conducted in patients requiring CABG who had severe MR or symptomatic moderate MR, ejection fractions >=25 %, and LVEDD < =7.0 cm. The hypotheses tested were that investigative “off-pump” treatment would have non-inferior efficacy (as measured by MR degree) and superior safety efficacy as compared to standard mitral repair [36]. The trial was terminated prematurely when the recent financial collapse resulted in the bankruptcy of the trial sponsor (Myocor Inc.).
Recruitment had accrued 165 patients, the prespecified value for the “first-look” data analysis. The Coapsys device was associated with greater long-term positive ventricular reshaping, with the LVEDD decreasing from 6.0 ± 0.8 to 5.4 ± 0.8 cm as compared to 5.9 ± 0.7 to 5.6 ± 0.9 cm for the control MV repair (effect of time p < 0.001, repeated measures analysis of variance [ANOVA]; effect of treatment p = 0.02). However, the MR treatment efficacy was not as effective with the Coapsys treatment: the standard mitral repair technique reduced MR (0 = none, 1 = mild, 2 = moderate, 3 = moderate-severe, 4 = severe scale) from 2.54 ± 0.80 to 0.35 ± 0.63 at 24 months, while Coapsys reduced MR from 2.40 ± 0.87 to 1.24 ± 0.97 (both effect of time and treatment p = 0.0001, repeated measures ANOVA). What was totally unanticipated was that the trial discerned a significant survival benefit to the Coapsys treatment; at 24 months, there was nearly half the incidence of death with the Coapsys device as compared to standard mitral repair (Fig. 4.1). Twenty-four-month survival from all cause death was 89 % in the Coapsys randomized group as compared to 78 % in the standard treatment group (adjusted log-rank 4.30; p = 0.038; intent-to-treat analysis); a more powerful benefit to the Coapsys treatment was noted in the as-treated analysis (p = 0.020).
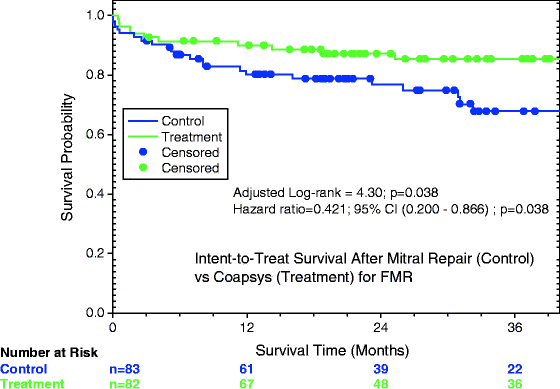

Fig. 4.1
Randomized Coapsys trial demonstrating late superior survival from all cause death for the Coapsys + CABG patients as compared to the control mitral repair + CABG patients (Reprinted from the Journal of the American College of Cardiology, Vol. 56, Grossi EA, Patel N, Woo YJ, et al., Outcomes of the RESTOR-MV Trial (Randomized Evaluation of a Surgical Treatment for Off-Pump Repair of the Mitral Valve), pp. 1984–1993, copyright 2010, with permission from Elsevier)
These findings are very provocative: patients with FIMR requiring revascularization treated with ventricular reshaping rather than standard mitral repair surgery had improved survival and significant reduction of major adverse outcomes. This unique dataset should guide further research in this area towards “ventricular solutions.”
Current Clinical Trials
Currently two topical clinical trials regarding the outcomes of ischemic mitral regurgitation are being conducted by the National Heart, Lung, and Blood Institute (NHLBI)-sponsored Cardiothoracic Surgical Trials Network. The first trial is entitled “Evaluation of Outcomes Following Mitral Valve Repair or Replacement in Severe Chronic Ischemic Mitral Regurgitation.” In this study, patients with severe FIMR will be randomized to either mitral repair or replacement; concomitant CABG will be performed if indicated. Pre- and postoperative evaluation will include cardiopulmonary exercise evaluation. The patients will be followed for 24 months. Interestingly, no restrictions are being applied as to the mitral valve repair technique employed by an individual surgeon.
The second trial is entitled “Surgical Interventions for Moderate Ischemic Mitral Regurgitation.” The purpose of this trial is to determine whether repairing moderate mitral insufficiency at the time of planned CABG will have beneficial effects. Again, cardiopulmonary exercise testing, neurocognitive tests, and quality of life surveys will be conducted over a 2-year period. Unfortunately, patient recruitment has been an issue for both trials. It has been speculated that there is a lack of clinical equipoise when treating “stentable” coronary artery disease in the presence of moderate MR which has limited patient referral. The NHLBI has announced a request for additional investigative sites to correct this issue.
Summary
FIMR is a common end-stage complication of coronary artery disease that develops from myocardial injury and subsequent negative LV remodeling. While various animal models have been developed to offer insight into this complex pathologic process, data inferred from them is conflicting. More sensitive and specific models are warranted to gain insight into patient-specific disease status and treatment outcomes.
FIMR can be eliminated with valve replacement or repair techniques, and this provides documented relief of heart failure symptoms. Notably, in patients with smaller ventricles, a majority will have positive LV remodeling. Mitral repair appears to have benefit over replacement for the majority of patients. However, in those patients who are NYHA class 4 or greater than 70 years of age, there is no advantage to repair over replacement. Extensive valvular distortion is probably best treated with mitral replacement. Novel techniques are being developed not only to treat the valve but also to treat the underlying ventricular disease. The combined approaches of annular repair and ventricular reshaping may offer the best therapy for this very sick patient cohort in the future.



