Etiology
Most cases of mitral stenosis (MS) are caused by rheumatic heart disease (Fig. 78–1).1 However, rheumatic fever has become quite rare in developed nations and so too has MS become rare. Indeed, most MS in the United States occurs in patients who have emigrated here from countries where rheumatic fever is still commonplace. Why rheumatic fever has waned in developed nations is unclear. Although antibiotic use almost certainly plays a role,2 the decline in disease incidence began before antibiotics were widely available, suggesting that socioeconomic factors also play a key role in the disease process. In addition, the organism responsible (group A Streptococcus) itself may have mutated to a less rheumatologic agent.
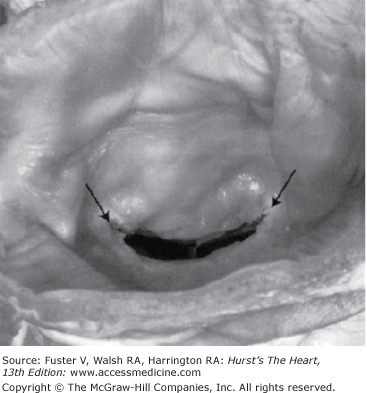
Figure 78–1.
The typical fish mouth appearance of rheumatic mitral stenosis is shown. Reproduced with permission from Otto.1
Although it is clear that rheumatic fever causes the disease, the exact mechanisms are still controversial. It is generally agreed that rheumatic fever occurs after infection with group A Streptococcus.3-5 The bodily defense against the organism attacks “M” protein antigens shared by the bacterium and the heart in some patients.4 Thus there is an inflammatory response that leads to cardiac damage, potentially of all three cardiac layers, the pericardium, myocardium, and endocardium. However, it is the endocardium, from which the cardiac valves are derived, that is most severely affected. Although all four valves may become damaged, the mitral valve is virtually always affected, but the reasons for this propensity are unclear. Perhaps greater mechanical stress on the mitral valve causes the inflammatory process to be manifested more severely there than on other valves.
The initial attack causes inflammation, thickening, and retraction of the mitral leaflets, usually causing mild mitral regurgitation, which may disappear as the attack subsides. Why MS develops later is not entirely clear, but at least 3 factors contribute to the process: sex, the severity of carditis in the first attack, and the number of subsequent attacks. Mitral stenosis is primarily a disease of women, with a 3:1 female preponderance. If after the initial attack there is little evidence of valvulopathy and no subsequent attacks occur, the chance that the patient will develop severe MS later in life is probably less than 5%.6 Subsequent attacks can be prevented by faithful adherence to antibiotic prophylaxis. However, what pathologic processes occur between the initial attack of acute rheumatic fever and eventual development of MS (when it does occur) are uncertain. At the time of surgery for MS there are active Aschoff nodules (the pathognomonic lesion of rheumatic fever) in the left atrial appendages of many patients, suggesting that a smoldering rheumatic process persists years after the last acute attack.7 Alternatively, it may be that after the initial lesion is created by rheumatic fever, hemodynamic stress on the valve may lead to continued inflammation and scarring. In fact, C-reactive protein is elevated in many MS patients, indicative of ongoing inflammation from either or both processes.8,9
Occasionally MS develops from nonrheumatic causes. Extensive annular calcification in the elderly may limit valve area.10 Approximately 50% of such patients progress to more severe disease over time. Radiation and restrictive mitral valve repair for mitral regurgitation are additional rare causes.11,12 At the other end of the age spectrum, congenital malformation is another cause of MS.
Pathophysiology
In normal subjects, mitral valve area is 4.0 to 5.0 cm2, such that in diastole there is hemodynamically a common chamber of the left atrium (LA) and left ventricle (LV). Thus as shown in Fig. 78–2A, there is an initial small gradient across the mitral valve that rapidly dissipates so that throughout most of diastole, pressures in the LA and LV are equal. However in MS, as the mitral valve becomes progressively narrowed, a gradient develops across the valve (Fig. 78–2B) so that LA pressure exceeds LV pressure.13 As MS worsens, LA pressure becomes progressively higher, in turn creating progressively more pulmonary congestion (the grades of MS severity as suggested in the American Heart Association/American College of Cardiology Guidelines for the Management of Valvular Heart Disease are shown in Table 78–1).14 The force needed to overcome the increased LA pressure and to drive blood past the stenotic mitral valve is generated by the right ventricle (RV), such that right ventricular pressure and pulmonary pressure become elevated. As MS becomes severe, secondary vasoconstriction in the pulmonary bed causes a further increase in pulmonary artery pressure, and pulmonary hypertension may become extreme. The exact cause of pulmonary vasoconstriction in MS is unknown. It is known that pulmonary hypertension is almost always reversed by relief of MS15 and also can be reversed by administration of phosphodiesterase inhibitors such as sildenafil16 or by nitric oxide inhalation.17 These data suggest that the nitric oxide pathway is in some way involved in the mechanism of pulmonary vasoconstriction and pulmonary hypertension in MS.
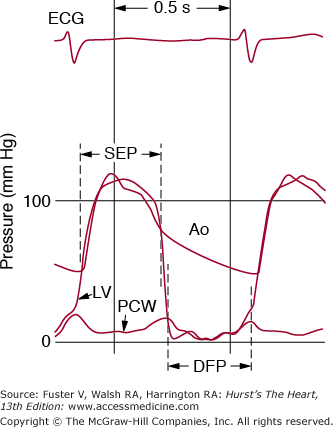
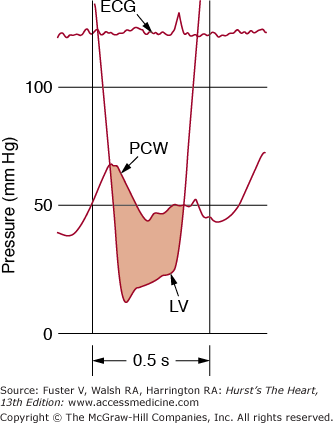
Figure 78–2.
A. Normal left ventricular (LV), left atrial (LA), and aortic (Ao) pressure tracings are shown. DFP, diastolic filling period; ECG, electrocardiogram; SEP, systolic ejection period. B. The pressure gradient between pulmonary capillary wedge pressure (PCW) and left ventricle (LV) is shown for a patient with MS. In this figure, the LV end diastolic pressure is atypically elevated, consistent with coincident mitral regurgitation. Reproduced with permission from Carabello and Grossman.13
| Mitral Stenosis | |||
|---|---|---|---|
| Mild | Moderate | Severe | |
| Mean gradient (mm Hg)a | Less than 5 | 5–10 | Greater that 10 |
| Pulmonary artery systolic pressure (mm Hg) | Less than 30 | 30–50 | Greater than 50 |
| Valve area (cm2) | Greater than 1.5 | 1.0–1.5 | Less than 1.0 |
It is generally held that MS “protects” the LV from damage. That is, unlike other left-sided valve lesions, MS creates no overload on the LV and in fact may decrease preload by impairing LV filling. It is of interest then that approximately one-third of patients with MS have reduced indexes of LV ejection performance.18-20 The mechanism of reduced LV ejection has been the subject of controversy for many years, and some have invoked a “myocardial factor” (myocardial damage from rheumatic fever) as the cause. Although rheumatic fever may cause LV damage in developing countries where the rheumatic process may be very aggressive,21 in developed countries, LV contractility in MS appears to be normal. Instead, reduced LV performance is caused by abnormal loading conditions. Unexpectedly, afterload may be abnormally high in some patients with MS.20 Increased afterload accrues in part from arterial vasoconstriction caused by sympathetic reflexes to decreased cardiac output. In addition, the LV in some MS patients remodels in such a way that the LV is thinner than normal, increasing wall stress (afterload).20,21 In most other cardiac diseases, increased afterload is offset by increased preload to maintain stroke volume. However, MS limits LV filling, preventing full utilization or preload reserve, and thus ejection fraction is reduced. Proof of this concept follows from the changes seen immediately after balloon mitral valvotomy, where acute relief of LV inflow obstruction results in rapid correction of LV ejection indexes, indicating that loading abnormalities rather than a myocardial factor must have been involved.22
Diastolic function in MS may also be abnormal. Reverse LV remodeling to smaller than normal volume and regional tethering caused by distortion of the subvalvular apparatus lead to abnormal LV compliance. Like the systolic abnormalities noted earlier, these are also reversed rapidly after balloon valvotomy.23
The pulmonary hypertension that develops in MS causes right ventricular pressure overload and eventual RV failure. Although the irregular shape of the RV makes assessment of contractility difficult, most studies have concluded that RV failure, like LV failure, in MS is mostly due to afterload excess rather than contractile dysfunction.24,25 This is in contradistinction to experimental RV pressure overload, where contractile dysfunction has been well documented.
Thus MS causes reduced cardiac output and pulmonary congestion typical of LV failure, yet these abnormalities are more the consequence of abnormal loading and LV remodeling rather than of myocardial dysfunction. Likewise, RV failure also seems to be a consequence of increased afterload rather than of myocardial dysfunction.
Clinical Presentation
Most patients with mild MS are asymptomatic. Indeed, many women are unaware that they have the disease until the increased cardiac demands of pregnancy cause symptoms to appear. As MS worsens, symptoms typical of left-sided heart failure occur, even though, as noted earlier, left ventricular contractility is often normal. Thus dyspnea on exertion, orthopnea, and paroxysmal nocturnal dyspnea are common. If pulmonary hypertension develops, ascites and edema may follow. A symptom seen in MS but rare in other causes of heart failure is hemoptysis, usually during activity. As predicted by the Gorlin formula,26 if exercise causes cardiac output to double, the transmitral gradient will quadruple, causing a sudden increase in LA pressure. This increase is thought to cause rupture of small pulmonary vein anastomoses, leading to frank hemoptysis to be distinguished from the pink frothy sputum seen in pulmonary edema. In cases where the LA becomes so large that it impinges on the left recurrent laryngeal nerve, hoarseness appears (Ortner syndrome).
Physical examination of the patient with MS may reveal a plethora of signs, many of which are subtle, so that the exam should take place in a quiet undisturbed setting. If the patient is experiencing rapid atrial fibrillation, many signs may be inaudible, obscuring the diagnosis. Cardiac examination may find the irregularly irregular pulse of atrial fibrillation, common in older patients with MS. The apex beat is usually in its normal position, but palpation may reveal a diastolic thrill when the patient is examined in the left lateral decubitus position. Palpation of the sternum may find an RV lift in the presence of pulmonary hypertension. S1 is typically quite loud because the transmitral gradient holds the valve open throughout diastole rather than allowing the valve to drift closed just before systole, as it normally does. Thus systole closes the valve from its fully open (albeit stenotic) position, creating a loud S1. However, in far advanced disease, the valve may have little motion and S1 becomes soft. S2 may be normal, or its P2 may be loud if pulmonary hypertension has developed. S2 is then followed by an opening snap (OS), and the S2-OS interval gives a good clue to the severity of the MS. As severity worsens, the LA pressure increases so that LA pressure exceeds LV pressure (the force that opens the valve) relatively soon after S2, and the S2-OS interval is short, approximately 60 ms, just a bit longer than the normal splitting interval of A2 and P2 during inspiration. Conversely, in mild disease, the S2-OS interval is long, approaching 120 ms, similar in cadence to an S3. It should be noted that S3 and S4, sounds produced by rapid LV filling, are typically absent in this disease that impairs the transfer of blood from LA to LV. After the OS, the typical low-pitched rumble of MS is heard best at the LV apex (Fig. 78–3).27 If the patient is in sinus rhythm, there may be presystolic accentuation of the murmur. Examination of the lungs may detect rales, although it is remarkable that some patients with very high LA pressure have clear lung fields upon auscultation. It is thought that lymphatic hyperfunction clears the alveoli of transudated fluid that would be expected to form from high LA pressure.28
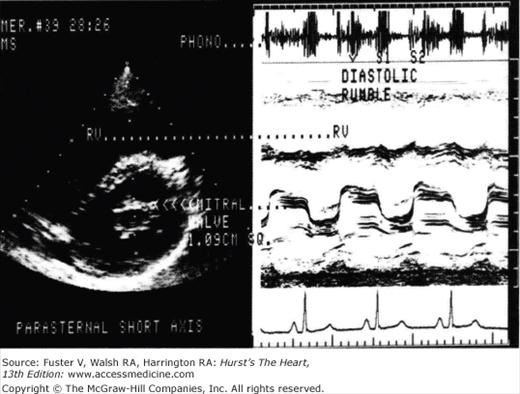
Figure 78–3.
The echocardiogram and phonocardiogram for a patient with mitral stenosis is shown. The phonocardiogram demonstrates the typical loud S1 opening snap and diastolic rumble. Reproduced with permission from Assey et al.27
If pulmonary hypertension has ensued, the high-pitched murmur of pulmonary insufficiency (Graham-Steell) may be heard in the pulmonic area. However, the concomitant presence of aortic insufficiency is often mistaken for this murmur.29 Neck vein distension, ascites, hepatomegaly, and edema reflect RV failure.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree