The systematic review and meta-analysis by Micha et al addresses a clinically important issue. Methotrexate is the most common disease-modifying antirheumatic drug prescribed in patients with rheumatoid arthritis (RA). Unfortunately, the validity of the review is compromised by misclassification of the epidemiologic design of the original studies and by several errors in the effect sizes and related 95% confidence intervals (95% CIs) extracted from them.
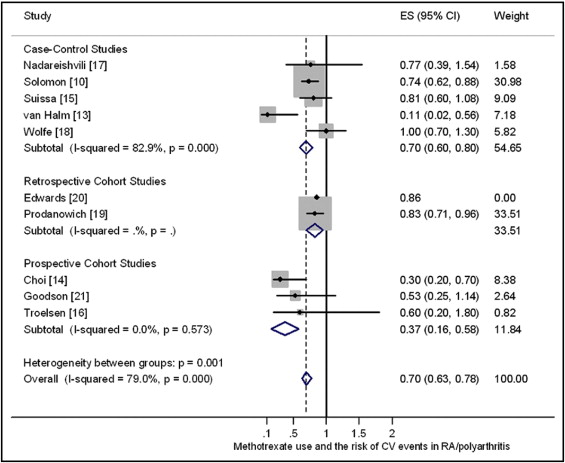
In the main summary table (Table 1), 7 of the 10 included studies are identified as prospective cohort studies. In fact, only 3 of the studies were prospective cohort studies; 5 were case-control studies, and 2 are retrospective cohort studies. In the main forest plot (Figure 2), the study by van Halm et al has an effect size of 0.53 (95% CI 0.24 to 1.19), but the original published value was 0.11 (95% CI 0.02 to 0.56). The study by Edwards et al, published as an abstract, has a 95% CI of 0.56 to 1.32, but this was not reported in the original study. There are also some relatively minor errors in presenting the 95% CIs from the studies by Choi et al, Wolfe et al, Suissa et al, and Prodanowich et al.
“Methotrexate monotherapy” was the reference group in the study by Solomon et al. The reviewers estimated the effect size by pooling the inverse effect sizes for the other RA drugs (biologics, other cytotoxic drugs, non–cytotoxic drugs, and glucocorticoids) to derive an effect size of 0.74 (95% CI 0.62 to 0.88) for inclusion in the meta-analysis. This involved pooling the effect sizes and 95% CIs from 4 multivariate models. Our attempt to replicate this approach from the original published data produced an effect size of 0.69 (95% CI 0.54 to 0.84). A more rigorous approach to estimating the fully adjusted effect sizes and 95% CIs would be to reanalyze the association in a single multivariate model using the original individual patient data. This might be feasible, as the lead investigator of this study is also a coauthor of this review.
The study by Prodanowich et al reported that some patients had RA and psoriasis. Because the RA and psoriasis arms of this study were included in the meta-analysis, it is likely that some patients were counted twice. The original study does not report the proportion of patients involved, but research from elsewhere suggests about 10%. Because psoriasis vulgaris and RA are also distinctly different clinical conditions, we think that the psoriasis arm of the study by Prodanowich et al should have been excluded from the meta-analysis.
Our forest plot ( Figure 1 ) shows a reanalysis of the data (fixed-effects model using the metan command in Stata version 10.1; StataCorp LP, College Station, Texas) for the 10 studies involving RA (excluding the psoriasis arm of the study by Prodanowich et al, but including the study by Goodson et al of early inflammatory polyarthritis, because a related report indicates that 90% of these patients will have RA after 3-year follow-up); with analysis stratified by study design and including the effect sizes and 95% CIs reported in the original studies. Our meta-analysis identified a much greater degree of heterogeneity (I 2 = 79% vs 15%) and a larger effect in reducing cardiovascular events (0.70 vs 0.79) compared to the published review. The source of this heterogeneity merits further investigation. For example, the assessed quality of the primary studies were generally low, and only 3 studies (Choi et al, Troelsen et al, and van Halm et al) ensured that all their patients fulfilled the American College of Rheumatology criteria for RA. These 3 studies demonstrated generally larger effects in reducing cardiovascular events than other studies, with a pooled effect size of 0.23 (95% CI 0.05 to 0.41).