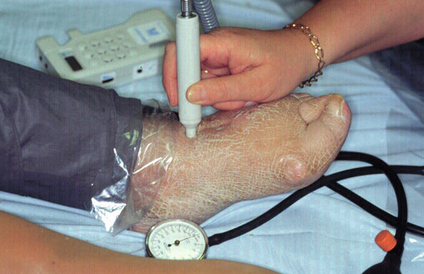
3 Peripheral arterial disease (PAD) is an extremely common condition. Patients with symptomatic PAD have reduced mobility and quality of life, which equates to some cancers, but PAD is also a powerful marker of cardiovascular risk, equating to a previous myocardial infaction.3 In a survey of 1886 patients with PAD, 58% had coronary artery disease and 34% had suffered a cerebrovascular event.4 In summary, PAD is present in over 29% of the adult population and in the majority is asymptomatic. Apart from the impact on the patient in those with symptoms, PAD also identifies patients at extremely high risk of cardiovascular events, particularly if they have arterial disease elsewhere (Fig. 3.1). The key aims of treatment of PAD should be reduction of cardiovascular risk and improvement of the symptoms. Despite these stark figures, evidence suggests that many patients with PAD remain undiagnosed and even those who have been identified get suboptimal treatment, especially when compared to patients with coronary artery disease.7 This chapter will address the diagnosis and detection of PAD, factors affecting cardiovascular risk and medical treatments that may improve the symptoms of PAD. In symptomatic patients the diagnosis of PAD can be made on structured questioning, examination and measurement of ankle–brachial pressure index (ABPI). Typical muscular pain in the calf or thigh and buttocks on walking, combined with absent lower limb pulses, is strongly suggestive of PAD (Fig. 3.2). However, many patients have comorbidities, such as arthritis, which can confuse the history and pulse palpation may be difficult. Based on the above clinical criteria alone PAD will be missed in many individuals, and objective methods of assessment are needed, especially in asymptomatic people.8 Measurement of ABPI has been shown to be a reproducible method of confirming the diagnosis and is widely applicable in primary and secondary care9 (Fig. 3.3). Figure 3.3 Measurement of ankle–brachial pressure index in a patient with PAD. The technique should be standardised. The patient should be supine and rested. The cuff size should be appropriate for the patients limbs and placed just above the malleoli. Using an 8-MHz Doppler ultrasound probe the systolic pressures in the anterior tibial (illustrated) post-tibial and peroneal arteries should be recorded. The pressures in both arms should be recorded. The ankle–brachial pressure index (ABPI) is the highest ankle pressure divided by the highest arm pressure. The observation that a reduced ABPI, even in asymptomatic patients, correlates with increased cardiovascular risk prompts the concept of screening the adult population.15 There is overwhelming evidence for the benefits of identifying and correcting risk factors such as hypertension, dyslipidaemias, diabetes and obesity in patients with PAD. Active intervention to aid smoking cessation, increased exercise as well as the use of antiplatelet agents will result in a marked reduction in cardiovascular morbidity and mortality (see Chapter 1). Despite such evidence the delivery of this aspect of medical treatment remains poor. In the ongoing REACH registry of patients at increased cardiovascular risk principally being managed in primary care, only a minority of patients with PAD receive adequate medical treatment.17 Even in patients referred to secondary care only 70% were on antiplatelet therapy and 44% taking a statin.18 Perhaps most disappointing of all, even after secondary care involvement there still remains a number of patients receiving inadequate treatment. In a retrospective review of 109 patients who had undergone amputation due to PAD at the time of referral for prosthesis fitting only 41% had been prescribed a statin and only 60% were taking an antiplatelet agent; 39% of patients were on both a statin and antiplatelet agent, but 32% had been prescribed neither.19 Lay knowledge of PAD too is poor compared to CHD and stroke. In a group of 2501 adult Americans only 26% had ever heard of PAD. Of those with awareness of PAD 56% were aware it was associated with smoking and approximately 25% were aware it was linked to MI, stroke and amputation.20 Secondary prevention clinics for CHD have been demonstrated to save lives and it seems reasonable to extrapolate this benefit to patients with PAD.21 Exercise is widely held to be of benefit to patients with PAD and not only improves walking distance but may also help reduce cardiovascular mortality. Unfortunately only 27% of vascular surgeons in the UK have access to such programmes.22 Although most studies comparing exercise with other therapies have been small and used different exercise regimens there is good evidence for the improvement in muscle function, vascular endothelial cell function and metabolic adaptations with exercise.23 In a randomised controlled trial of three treatments for intermittent claudication due to femoropopliteal disease, angioplasty, supervised exercise or a combination of angioplasty and supervised exercise, all treatments improved walking distance and quality of life (QOL) at 12 months. The combination of exercise and angioplasty tended to gain more improvement in the short term than either treatment alone but the QOL gain was equal in all groups.26 The MIMIC Trial also showed a benefit of combining supervised exercise, best medical therapy and angioplasty over best medical therapy and angioplasty for patients with either superficial femoral artery disease or aortoiliac disease.27
Medical treatment of chronic lower limb ischaemia
Introduction
PAD diagnosis and screening
Modifying cardiovascular risk
Medical treatments for symptomatic PAD
Exercise
< div class='tao-gold-member'>
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Medical treatment of chronic lower limb ischaemia
Only gold members can continue reading. Log In or Register to continue


