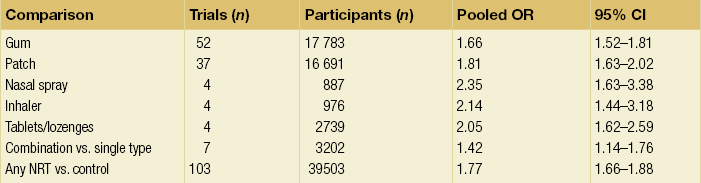
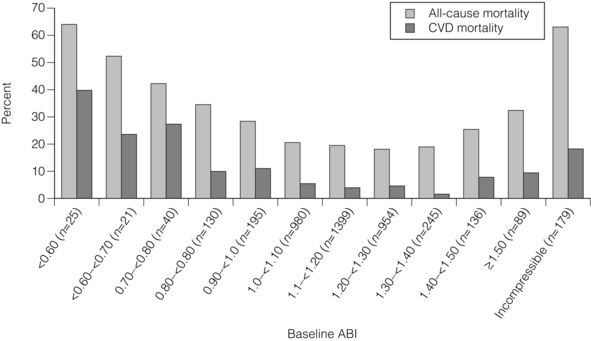
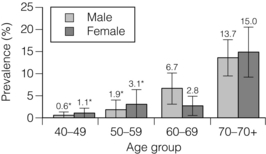
1 Atherosclerotic peripheral arterial disease (PAD) involving one or more major vessels of the lower limb is common, especially in older patients, due to complex genetic and environmental interactions that result in structural and functional vascular abnormalities and reduced blood flow. PAD may be asymptomatic in the early stages, but is always associated with shortened survival due to the invariable association with atherosclerosis in other arterial territories, especially the coronary, carotid and cerebral circulation. This is highlighted by observational studies showing that reduced ankle–brachial pressure index (ABPI, a marker of disease severity in PAD) is associated with an increased risk of cardiovascular mortality (Table 1.1).1 However, calcification and sclerosis lead to incompressible arteries, with false elevation of ABPI even in the presence of major distal atherosclerosis. The Strong Heart Study has identified associations between low (<0.90) and high (>1.40) ABPI and increased risk of all-cause and cardiovascular (CV) disease mortality, reporting a U-shaped relationship between a non-invasive measure of PAD and reduced life expectancy (Fig. 1.1).2 For example, adjusted risk estimates for all-cause mortality were 1.69 for low and 1.77 for high ABPI, while the corresponding estimates for CV disease mortality were 2.52 and 2.09.2 Table 1.1 Adjusted relative risk for mortality for levels of ankle–brachial pressure index (ABPI) From McKenna M, Wolfson S, Kuller L. The ratio of ankle and arm arterial pressure as an independent predictor of mortality. Atherosclerosis 1991; 87:119–28. With permission from Elsevier. Figure 1.1 Relationship between ABPI (ankle–brachial pressure index) and survival in patients in the Strong Heart Study.2 There is a U-shaped relationship such that both low (<0.9) and high (>1.4) ABPI is associated with increased risk of CV and all-cause mortality. Obtaining accurate figures for the prevalence and incidence of PAD has not been straightforward. For example, several epidemiological studies have focused on specific groups, e.g. in the workplace setting or referrals to hospital, which may not be truly representative of the wider population. Thus, workplace screening studies for PAD have excluded those who have retired and those who may be unfit for work. Similarly, epidemiological studies based on inpatient or outpatient referrals tend to underestimate the prevalence of PAD in the community. One of the largest and most reliable sources of information about the overall prevalence of symptomatic and asymptomatic PAD is the Edinburgh Artery Study, which screened large random samples of the general population using age/sex registers from general practices.3,4 Clearly, the technique used to establish the presence or absence of PAD will also affect the results of epidemiological surveys. Questionnaires have often been used to establish the nature and severity of symptomatic PAD, e.g. the WHO/Rose questionnaire designed in 1962. The original questionnaire developed by Rose was shown to be highly sensitive but only moderately specific, and therefore in 1985 the tool was modified in a way that increased the specificity, albeit at the expense of a small decrease in sensitivity.5 The Edinburgh Artery Questionnaire is designed to be self-administered and has a sensitivity of 91% and a specificity of 99% for symptoms of PAD.6 In general, all questionnaires appear to underestimate the true prevalence of intermittent claudication and the Transatlantic Inter-Society Consensus (TASC) group recommend great caution in interpreting epidemiological studies of symptomatic PAD based solely on questionnaires. Physical examination to establish the presence or absence of peripheral pulses has also been used in epidemiological surveys to confirm a history of intermittent claudication. However, the absence of a peripheral pulse is not necessarily due to PAD, and at least one pulse may be undetectable in up to 10% of the adult population even though only 3% have symptomatic arterial disease.7 Establishing the prevalence of asymptomatic PAD in the general population is equally important. The most useful non-invasive test for this purpose is the ABPI, which is quick and painless and has excellent sensitivity and specificity. An ABPI <0.9 is 95% sensitive and 100% specific for detecting angiogram-positive disease.8 At the more severe end of the spectrum, most of the data on the prevalence of critical limb ischaemia has been obtained from inpatient records, and only rarely from population-based studies or using ABPI criteria. Evidence from epidemiological studies using ABPI suggests that the prevalence of asymptomatic PAD in the middle-aged and elderly population is around 7–15%.3,9 However, in the British Regional Heart Study, direct assessment of the femoral artery with ultrasound found that 64% of people aged 56–77 years had significant femoral atherosclerosis and only 10% of these were symptomatic.10 Autopsy studies have found similar results, suggesting that the true incidence of asymptomatic PAD may be much higher than previously recognised. Population studies have varied widely in reporting the incidence of intermittent claudication. Most of these are based on questionnaire surveys and therefore prone to some degree of over-reporting. Nevertheless, it is clear that the incidence of intermittent claudication increases steeply with age. The Scottish Heart Study, for example, found a prevalence of 1.1% in subjects aged 40–59 years;11 in the Limburg study (subjects aged 40–79 years) the reported prevalence varied between 1.4% and 6.1%12 depending on the criteria used, and the Edinburgh Artery Study indicated a higher prevalence of 4.5%, but in a group (55–74 years) with older mean age.3 Information about the prevalence of PAD in the USA has emerged from the National Health and Nutrition Examination Survey (NHANES, 1999–2000).13 By analysing data from 2174 participants, Selvin and Erlinger found that, among adults aged 40 years and over, the prevalence of PAD was 4.3% (PAD was defined as ABPI <0.90 in either leg). This equates to approximately 5 million people in the USA with PAD. Among those over 70 years old, the prevalence was 14.5%13 (Fig. 1.2). Figure 1.2 Recent information about the prevalence of PAD from the US National Health and Nutrition Survey, confirming a steep age-related prevalence.13 The incidence of critical limb ischaemia has been estimated to be around 400 cases per million population per year, which equates to a prevalence of 1 in 2500 of the population annually.14 For every 100 patients with intermittent claudication, approximately one new patient per year will develop critical ischaemia.8 The Edinburgh Artery Study is one of the few studies to have examined the pattern of progression among asymptomatic patients with abnormal ABPIs and the rate of development of symptoms; 7–15% of subjects with asymptomatic PAD developed intermittent claudication over a 5-year period, depending on the initial severity of the disease.4 A more recent study from the Netherlands reported similar conversion rates, with 27 of 177 asymptomatic patients (15%) developing lower limb symptoms during a 7-year follow-up period.15 Information about longitudinal changes in ABPI, and risk factors for declining ABPI, has emerged from the Cardiovascular Health Study.16 Among 5000 patients with normal ABPI at baseline, 9.5% had a significant decrease in ABPI during a 6-year follow-up. Independent predictors of ABPI decline included age (odds ratio (OR) 1.96 for the 75–84 age group and 3.79 for those >85 years), current cigarette use (OR 1.74), hypertension (OR 1.64), diabetes (OR 1.77) and raised low-density lipoprotein (LDL) cholesterol.16 Reduced ABPI has also been associated with rising serum creatinine,17 indicating that even asymptomatic PAD may affect renal outcomes. There is good evidence that subjects with asymptomatic PAD have a much higher risk of systemic CV complications. The risk of death or disability from cardiac or cerebral events may be much higher than the risk of lower limb symptoms (claudication or acute limb ischaemia). The Edinburgh Artery Study showed that asymptomatic PAD patients have an increased risk of acute myocardial infarction and stroke; in fact, they have almost the same increased risk of CV events and death as that reported among patients with claudication.3 The reverse also applies, e.g. in men with asymptomatic carotid stenosis ABPI was the strongest predictor of stroke risk.18 Large population follow-up studies suggest that up to 50% of patients with intermittent claudication will remain relatively stable (i.e. no deterioration in walking distance) or experience some spontaneous improvement in symptoms during a 5-year period; only 25% of claudicants will develop significant deterioration in walking distance.19,20 The Basle study20 is typical of several observational follow-ups in showing that, although two-thirds of patients surviving at 5 years reported no limiting intermittent claudication (i.e. their symptoms had resolved), 63% actually had angiographic progression of the disease. This suggests that although PAD is pathologically progressive, other factors contribute to symptomatology, e.g. collateral vessel formation or physiological and psychological adaptation. Although one-quarter of patients with intermittent claudication have symptoms that worsen over time, only 5% deteriorate sufficiently to merit revascularisation and only 1–2% will require a major amputation.8 Although lower limb outcomes are mostly very good for patients with uncomplicated intermittent claudication, the major concern for these patients relates to a heightened risk of CV complications due to silent or symptomatic atherosclerosis in other vascular territories. Patients with intermittent claudication have a 2–4% risk of undergoing a non-fatal CV event within the first year of diagnosis and a 1–3% yearly incidence thereafter.8 For most patients, however, absolute coronary heart disease (CHD) risk is greater than 30% over 10 years, and all-cause mortality rates are similar to those associated with many forms of cancer. In the CASS study, patients with PAD had a 25% greater likelihood of mortality than patients without PAD.21 A national survey conducted in 1993 by the Vascular Surgical Society of Great Britain and Ireland found that around 70% of patients with critical ischaemia were offered some form of revascularisation procedure, with a 75% chance of limb salvage. The overall amputation rate, however, was still 21.5% and the mortality rate was 13.5%.14 Thus, the overall long-term prognosis for these patients is very poor. This large multinational registry is providing useful observational data about the spectrum of disease progression, CV outcomes and patterns of treatment in the 21st century. A total of 67 888 patients, aged 45 years or more, from 44 countries were registered in the database if they had either established CV disease or if they were asymptomatic with more than three risk factors (n = 12 389). Among the symptomatic group, patients were enrolled on the basis of coronary artery disease (CAD; n = 40 248), cerebrovascular disease (CVD; n = 18 843) or PAD (n = 8273);22 16% of this group had polyvascular disease. Three-year outcome data for the REACH cohort have been published.23 Among patients with established CV disease, CV death, myocardial infarction (MI) or stroke rates were 23.0% for patients with CAD, 18.7% for patients with CVD and 33.6% for patients with PAD. Patients with PAD were most likely to have subsequent vascular death (2.9 events per 100 person-years). There are numerous CV risk factors associated with atherosclerotic disease progression (Fig. 1.3). However, it is important to emphasise the distinction between a CV risk factor and the evidence that intervention to modify that risk factor improves clinical outcomes (i.e. symptoms or survival). Factors such as high serum homocysteine24 or fibrinogen concentrations may be weak risk factors for PAD, but for clinicians in practice this is largely meaningless without evidence from randomised prospective trials that lowering of homocysteine or fibrinogen affects disease outcome. Indeed, the point is well illustrated by a recent placebo-controlled trial of folic acid and vitamin B (B6 and B12) supplements, which achieved the aim of producing a sustained reduction in plasma homocysteine levels in patients with established CV disease, but the intervention had no effect on clinical end-points such as mortality or non-fatal CV events.25 In the study, using age- and gender-adjusted logistic regression analyses, the following risk factors (and odds ratios) were significantly associated with PAD: black race/ethnicity (OR 2.83), current smoking (OR 4.46), diabetes (OR 2.71), hypertension (OR 1.75), hypercholesterolaemia (OR 1.68) and poor kidney function (OR 2.00). Elevated fibrinogen and C-reactive protein levels also were associated with PAD.13 A similar profile of risk factors for PAD has been defined for patients with diabetes using the Atherosclerosis Risk in Communities (ARIC) study database.26 A recent study indicated that high serum adiponectin is a protective for the development of PAD in women.27 There is clear evidence from several studies that increasing age is associated with an increased risk of PAD in both men and women3,28 (Fig. 1.2). The evidence for a gender difference is slightly less clear. Several studies, including the Framingham Heart Study, have suggested that men have nearly double the risk of developing intermittent claudication compared with women,28 but the Edinburgh Artery Study failed to show any significant difference between the sexes,3 and a follow-up to the Limburg study suggested that the incidence of both symptomatic and asymptomatic PAD was greater in women.15 Family history is an independent risk factor for premature CHD, but studies have failed to show the same (presumably genetic) association for PAD. Cigarette smoking is associated with excess premature deaths from CV, respiratory and cancer-related diseases. Smoking is undoubtedly the most important modifiable risk factor for PAD. The relationship between smoking and lower limb arterial disease was first identified in 1911 by clinicians who reported a threefold increase in the incidence of claudication among smokers. Smoking not only affects the development of PAD but also affects the clinical outcome in those patients with PAD who continue to smoke. Smokers with PAD are much more likely to progress to critical ischaemia, and more likely to require an amputation or vascular intervention.29 Furthermore, smoking increases the overall mortality rate among claudicants by a factor of 1.5–3.0.6 Diabetes mellitus is well recognised as an important risk factor for cardiovascular disease and, apart from smoking, is probably the single most important risk factor in the development of PAD. Lower limb arterial disease tends to be more diffuse and distal in diabetics, with both ischaemic and neuropathic ulceration being common. The Edinburgh Artery Study and the Health Professionals Follow-up Study showed that people with diabetes have a 1.5- to 2.5-fold increased risk of symptomatic and asymptomatic PAD compared with non-diabetics, and the lifetime risk of a lower limb amputation is increased 10–16 fold.36,37 Duration of diabetes is also important, e.g. in the Health Professionals Follow-up Study patients were grouped according to diabetes duration and the relative risks of PAD were 3.63 (diabetes duration 6–10 years), 2.55 (11–25 years) and 4.53 (duration >25 years) compared with patients diagnosed less than 5 years.37 Several studies in both type 1 and type 2 diabetes have identified the glucose level as an independent risk factor for PAD.39,40 However, other important features of the ‘metabolic syndrome’ of type 2 diabetes include insulin resistance, hypertension, obesity and dyslipidaemia (typically low high-density lipoprotein (HDL) cholesterol and high triglycerides). Epidemiological data have suggested that both insulin resistance and hyperinsulinaemia are independent risk factors for PAD in diabetic and non-diabetic individuals.41 Hypertension plays a significant role in the development of PAD in diabetic subjects. The Hypertension Optimal Treatment (HOT) trial showed that vigorous blood pressure control had a greater effect in reducing CV events in those patients with diabetes than those without,43 and effective control of hypertension may limit vascular events even more effectively than tight glycaemic control.44 The major benefits of glycaemic control appear to be in microvascular protection and prevention of neuropathy and secondary foot complications such as ulceration and infection. The association of traditional and non-traditional risk factors with PAD incidence in a population of patients with diabetes has been investigated in the ARIC study.26 This analysis showed that patients with diabetes were more likely to develop PAD if they were smokers (relative risk (RR) 1.87), had CHD at baseline (RR 2.27) and high triglycerides (RR 1.75). Patients taking insulin therapy were also at higher risk.26
Epidemiology and risk factor management of peripheral arterial disease
Introduction

Epidemiology of PAD
Investigative techniques for epidemiological screening
Prevalence and incidence of PAD
Natural history of PAD: cardiovascular and lower limb outcomes
Asymptomatic disease
Intermittent claudication
Critical limb ischaemia
The Reduction of Atherothrombosis for Continued Health (REACH) registry
Epidemiological risk factors for PAD and randomised trials of disease-modifying therapy for secondary prevention
Risk factors for PAD
Age and gender
Cigarette smoking
Diabetes
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree