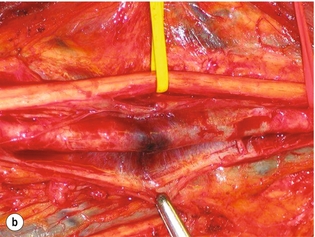
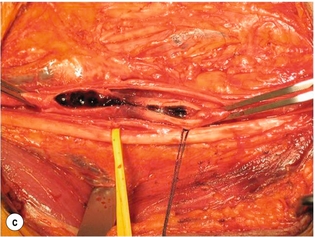
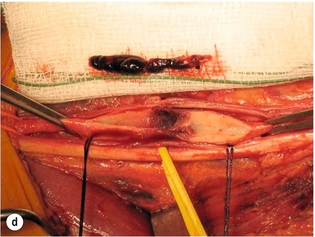
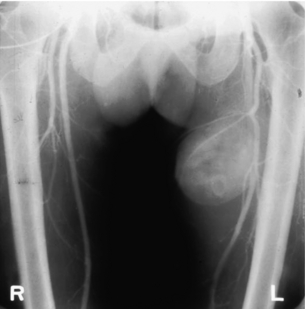
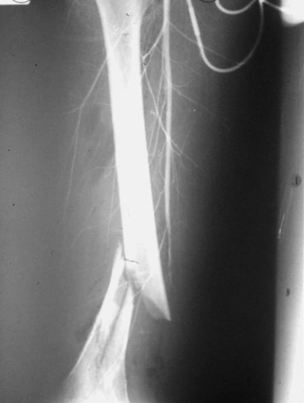
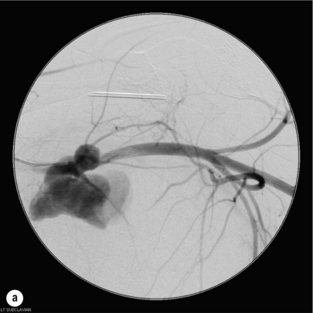
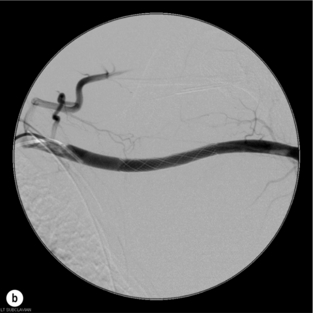
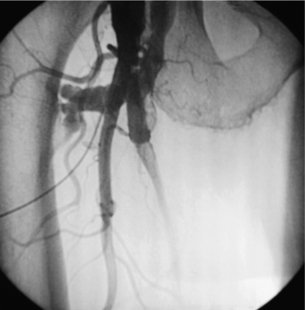
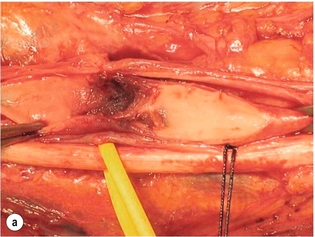
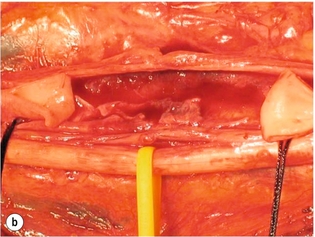
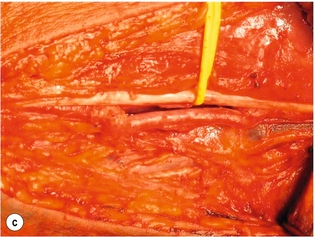
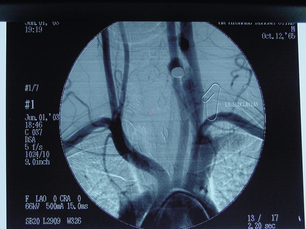
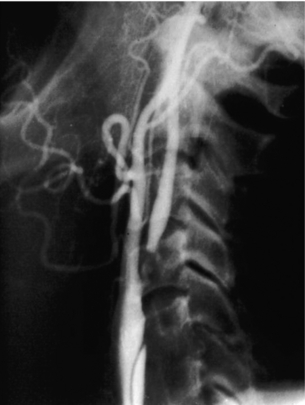
9 Fewer than 10% of patients with polytrauma have associated vascular injuries, but these injuries can cause significant morbidity and mortality.1 In most European countries the majority of vascular trauma is caused by blunt (traffic accidents) and iatrogenic injuries.2 In South Africa, injuries are mostly penetrating and have also changed from predominantly stab wounds to injuries caused by firearms.3 Vascular injuries are classified according to the mechanism of the injury. Direct trauma to the artery accounts for the majority of blunt vascular injuries. Indirect trauma is usually the result of shearing and distraction forces following dislocation of major joints, displaced long-bone fractures and acceleration/deceleration injuries as seen with high-speed motor vehicle accidents and falls from a height. Blunt trauma causes contusion of the arterial wall with disruption of the intima. This intimal tear may cause immediate obstruction due to an intimal flap or may predispose to thrombosis and delayed occlusion (Fig. 9.1a–d). As the vessel is stretched further, progressive layers of the media are disrupted until the continuity of the vessel is maintained only by the elastic adventitia or there is complete disruption. Penetrating trauma may result in partial or complete transection of a vessel. Bleeding is often brisk and distal flow may be interrupted. Stab and low-velocity missile injuries cause localised damage confined to the injury tract. High-velocity missiles cause total tissue destruction around the missile tract, surrounded by an area of doubtful tissue viability, causing extensive associated soft-tissue trauma. The shock wave of a high-velocity missile can also cause intimal injury.4 The vessel may be macroscopically intact with minimal bruising, but on opening the vessel there is an intimal tear with superimposed thrombosis. Shotgun injuries cause extensive local tissue destruction with often multiple sites of perforation. Bomb blasts cause complex injuries due to the combination of extensive local tissue trauma, high-velocity fragments and thermal injury. Iatrogenic injuries are becoming increasingly important and account for more than 40% of vascular trauma in many European countries.2 Vascular injuries have significant sequelae (Box 9.1, Figs 9.2 and 9.3). A contused artery may be patent initially but thrombose later. Subsequent propagation of thrombus may cause progressive ischaemia by obstructing essential collaterals. Acute ischaemia leads to degeneration and necrosis of muscle cells and Wallerian degeneration in nerves. Findings from large-animal studies indicate that early restoration of flow within 3 hours is associated with near-complete recovery, whereas delayed revascularisation at 6 hours was associated with significant muscle necrosis and nerve degeneration.5 Figure 9.2 Arteriovenous fistula of the right femoral vessels following iatrogenic injury after diagnostic cardiac catheterisation. Initial assessment should be carried out according to advanced trauma life support (ATLS) principles and life-threatening conditions managed. Vascular injury may present with any of the sequelae listed in Box 9.1. Clinical signs of vascular injuries can be divided into hard and soft signs. Hard signs of vascular injury: • Shock with ongoing bleeding. • Symptoms and signs of acute ischaemia. • Expanding or pulsating haematoma. Soft signs of vascular injury: • Injury of anatomically related structures. • Small non-expanding haematoma. • Multiple fractures and extensive soft-tissue injury. Plain radiographs are usually taken for associated skeletal injuries. A high index of suspicion for vascular trauma should exist with dislocations and displaced fractures (Fig. 9.4). Chest radiography is valuable in patients with chest trauma. Surgical intervention should not be delayed by arteriography where vascular injury is evident and the patient unstable or the limb is at ischaemic risk. On-table arteriography in the operating room should be performed in vascular injuries to the extremity where surgery cannot be delayed and the additional information is considered valuable.10 In blunt and high-velocity trauma there is often extensive intimal damage, and careful debridement of the vessel is necessary until normal-appearing intima is found (Fig. 9.5). Antegrade and retrograde flow should be evaluated. Arteries are cleared of thrombus by careful passage of embolectomy catheters followed by irrigation with heparin/saline solution. Figure 9.5 Blunt injury of the intima (a), resected (b) and replaced with a venous interposition graft (c). Simple laceration of the vessel wall is repaired by lateral suture, provided it does not lead to stenosis, when patch graft angioplasty is indicated. Where more than 50% of the circumference of a vessel wall is damaged, this area should be excised followed by end-to-end anastomosis. This requires mobilisation of the proximal and distal arterial stumps to achieve approximation without tension. Failing this, an interposition graft is indicated. Autogenous vein is the preferred conduit for reconstruction. Prosthetic material should only be used in exceptional circumstances due to the risk of graft infection.11 Endovascular techniques are used to manage vascular trauma in three ways: 1. To obtain haemostasis. Damaged vessels are embolised using a variety of substances including haemostatic agents (gel foam), coils and balloons. This technique has become the standard treatment for managing significant bleeding following pelvic fractures, and is also a recognised option for treating lesions of non-essential, inaccessible vessels in the cervical, pelvic and limb regions.13,14 It is also used to control bleeding due to penetrating and blunt trauma of the liver, kidneys and spleen.15–17 2. To obtain vascular control. Temporary balloon occlusion of a damaged vessel at the time of diagnostic angiography can prevent exsanguinating bleeding until surgical control is achieved. It is especially valuable in relatively inaccessible regions and allows limiting the extent of the exposure to obtain surgical control.18 This technique is valuable in injuries in zones 1 and 3 of the neck, the abdominal aorta, proximal subclavian and iliac arteries. 3. For vascular repair. Covered stent grafts are used for repairing vessels in anatomically challenging locations and to avoid major surgical exposures, e.g. the thoracic aorta, thoracic outlet vessels, internal carotid and vertebral arteries (Fig. 9.6).19–22 This will be discussed in more detail in the relevant sections. Covered stentgrafts may also be used as a temporary measure to allow stabilisation of the patient and definitive open repair later. The cervical vessels are involved in 25% of patients with head or neck trauma. Carotid artery injury constitutes 5–10% of all arterial injuries.23 The mortality for carotid injuries ranges from 10% to 31%, with permanent neurological deficit ranging from 16% to 60%.24,25 Penetrating injury may cause partial or complete transection of the vessel, pseudoaneurysm or arteriovenous fistula (Fig. 9.7). Pseudoaneurysm may have an acute or delayed onset, with progressive enlargement causing compression of the aerodigestive tract or brachial plexus. Blunt trauma may cause intimal flaps, intramural haematoma, dissection, complete disruption of arterial wall with pseudoaneurysms, arteriovenous fistulas and total occlusion (Fig. 9.8). Figure 9.7 Arteriovenous fistula between the carotid artery and internal jugular vein caused by gunshot wound. Figure 9.8 Dissection of the common carotid artery with blunt trauma to the neck following a motor vehicle accident. Active external bleeding, rapidly expanding cervical haematoma, absent carotid pulse and a bruit or thrill are indicative of vascular injury. Signs that may indicate an associated vascular injury warranting further investigation include bleeding from wounds of the neck or the pharynx, a deficit of the superficial temporal artery pulse, ipsilateral Horner’s sign, dysfunction of cranial nerves IX–XII, a widened mediastinum, fractures of the skull base and temporal bone, and fractures and dislocation of the cervical spine. Neurological deficit may be present, but obscured due to concomitant head injury, shock or the use of alcohol or drugs. About 50% of patients with established blunt injury to the carotid and vertebral arteries could initially be asymptomatic, but 43–58% of these will eventually develop neurological signs after hospital admission.26 The neck has been divided into three anatomical zones in order to standardise diagnosis and management of cervical vascular injuries (Fig. 9.9). Duplex scanning has limitations in zones 1 and 3, where arch angiography remains the gold standard for the diagnosis of vascular injuries. It is also important for proper planning of the surgical procedure and evaluation for possible endovascular treatment. Routine angiography is not indicated in asymptomatic patients.29 Computed tomography (CT) of the brain should be used to investigate patients with associated head trauma, bone injuries of the spine and skull, and neurological deficit. It is a good predictor of outcome: patients who have an infarct on initial CT on admission have a high mortality with poor chance of neurological recovery compared with those who have a normal CT on admission. MRA may be valuable in carotid artery and vertebral artery dissection.30 Active external bleeding can be controlled in the emergency room by direct digital compression or a Foley catheter inflated in the wound tract to obtain balloon tamponade.31 Mandatory exploration of all penetrating neck injuries has been replaced by a selective approach.32 Active pulsatile haemorrhage, expanding cervical haematoma and airway compromise are indications for urgent surgical exploration. Some low-velocity penetrating injuries may be managed expectantly with careful observation, provided there is no active bleeding and the distal circulation is normal.33 These injuries include intimal defects, small pseudoaneurysms (< 5 mm) and non-obstructive intimal flaps. The majority of penetrating carotid artery injuries, however, are best managed by primary arterial repair.34 Neurological deficit is only a contraindication to surgical repair in a deeply comatose patient with a dense neurological deficit, arterial occlusion and a huge infarct on cerebral CT.34 All other patients with associated neurological deficit would benefit from arterial repair, with improved mortality and final neurological status. Most blunt injuries of the carotid and vertebral arteries result in intimal disruption, with dissection and/or thrombosis, and the immediate goal of management is to restore cerebral perfusion and to prevent embolisation. Systemic anticoagulation is therefore the treatment of choice, because it limits the formation, propagation and/or embolisation of the thrombus. Intravenous heparin is administered in the acute phase, followed by oral anticoagulation for at least 3 months.35 Detailed description of operative technique falls outside the scope of this chapter and the reader is referred to the standard textbooks on operative surgery.36 The general principles of management include the following: • The patient should be in a supine position with a bolster between the scapulae and with the neck extended and the head rotated to the contralateral side. The patient must be draped to allow access from the base of the skull to the xiphisternum. • Zone 2 injuries are explored by the standard carotid incision overlying the anterior border of the sternocleidomastoid muscle. • Zone 1 injuries may require a median sternotomy. • Various techniques have been described to improve exposure of the distal internal carotid artery in zone 3 injuries, including subluxation of the mandible, mandibular osteotomy, excision of the styloid process, etc. • Some authors recommend routine shunting to maintain antegrade flow. • Where simple repair is not feasible, a bypass should be performed. Saphenous vein should preferably be used in the internal carotid artery whereas polytetrafluoroethylene (PTFE) is used to repair the common carotid artery. • The external carotid artery can be safely ligated if the internal carotid artery is patent. Internal carotid artery ligation is only recommended when the distal vessel is thrombosed with no back-bleeding following extraction of thrombus. • Minor venous injuries can be managed by lateral suture repair, but complex venous repair is not indicated as there is a high occlusion rate and it increases the magnitude of the operative procedure. Ligation of the jugular vein can be performed without significant sequelae.37 • In the presence of associated injuries to the trachea and oesophagus, the vascular repair should be protected by soft-tissue interposition (sternocleidomastoid muscle).
Vascular trauma
Introduction
Mechanism of injury
Blunt trauma
Penetrating trauma
Sequelae of vascular injuries
Clinical assessment
Examination
Special investigations
Arteriography
General principles of management of vascular injury
Endovascular management of vascular trauma
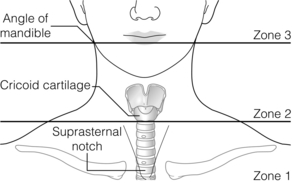
Cervical vascular injuries
Mechanism
Clinical signs
Diagnosis
Management
Operative technique
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Vascular trauma
Only gold members can continue reading. Log In or Register to continue