Historical Overview
Reperfusion therapy for acute myocardial infarction (MI) had its inception with Fletcher et al’s1 initial treatise describing the use of intravenous (IV) thrombolytic therapy in patients with thromboembolic disorders, including MI. Shortly after this, Boucek and Murphy2 published their observations of using catheters to deliver fibrinolytic therapy to the aortic root of patients presenting with acute MI, and Favaloro et al3 applied saphenous vein aortocoronary bypass surgery to patients presenting with acute infarction. Two groups, one in Spokane, Washington, and one in Göttingen, Germany, performed emergency catheterization before surgical revascularization for acute MI, and for the first time, knowledge of the coronary anatomy during acute MI became available.4,5 DeWood et al6,7 described the high prevalence of total coronary occlusion in the early hours after acute transmural MI and defined the role of the electrocardiographic (ECG) injury current in identifying a population of patients most likely to have acute total occlusion of the infarct artery and thus most likely to benefit from emergency revascularization.
In 1978, Rentrop et al8,9 performed emergency guidewire recanalization of an acute thrombotic coronary occlusion and subsequently reported on the first 13 patients with acute MI treated with mechanical reperfusion. These investigators reported their selective catheter infusion of intracoronary streptokinase results at the American Heart Association meetings in 1979, and the modern era of reperfusion therapy was born.10 When the works of DeWood and Rentrop were disseminated, enormous research interest in reperfusion therapy was generated in both Europe and the United States, and a number of randomized trials quickly followed. Khaja et al11 first demonstrated the efficacy of intracoronary streptokinase administration in establishing coronary reperfusion, and the Western Washington investigators12 documented improved survival in patients with acute MI treated with intracoronary streptokinase therapy. Because of the necessity of selective coronary angiography for this treatment, it quickly became apparent that a severe residual stenosis persisted in most patients after successful fibrinolysis. O’Neill et al13 demonstrated that balloon angioplasty could effectively treat the residual stenosis and that this resulted in less recurrent ischemia and better preservation of ventricular function. This report was the first to suggest an advantage of balloon angioplasty over fibrinolytic therapy. Logistical constraints and the limited number of trained operators and catheterization facilities hindered the development of both intracoronary streptokinase and primary angioplasty as reperfusion strategies in the mid-1980s.
The large GISSI (Gruppo Italiano per lo Studio del la Sopravvivenza nell’Infarto Miocardico) and ISIS (International Study of Infarct Survival)-2 trials,14,15 published in 1984 and 1986, definitively established the efficacy of IV streptokinase in improving survival in patients with acute MI. IV streptokinase with aspirin gained widespread use and became the standard of care as reperfusion therapy for patients with acute MI. Research interest soon focused on the development of new fibrin-specific fibrinolytic drugs that could be administered IV. However, many investigators were still concerned about the severe underlying residual stenosis remaining after medical reperfusion therapy, and three major randomized trials, the TAMI (Thrombolysis and Angioplasty in Myocardial Infarction), TIMI (Thrombolysis in Myocardial Infarction) II-A, and European Cooperative trials, were designed to determine the value of routine percutaneous transluminal coronary angioplasty (PTCA) after thrombolytic therapy.16-18 These studies gave surprising and disappointing results. Not only was routine PTCA unnecessary; it actually appeared harmful, and balloon angioplasty was mostly abandoned as an immediate adjunct to reperfusion with fibrinolytic therapy in the 1990s.
However, interest still persisted in the use of PTCA without antecedent fibrinolytic therapy (primary PTCA). Large credit should be given to the pioneering work of Hartzler et al19 in Kansas City, Kansas, who demonstrated the feasibility of primary PTCA.20 At the same time, Brodie et al21 in Greensboro, North Carolina, as well as O’Neill et al22 and Grines et al23 in Royal Oak, Michigan, concluded that primary PTCA had been inadequately tested as a reperfusion modality. These three groups joined forces and organized the original PAMI (Primary Angioplasty in Myocardial Infarction) study group. This group as well as investigators from the Zwolle group and the Mayo Clinic published the results of the first large randomized trials comparing primary PTCA with fibrinolytic therapy in 1993.24-25 These trials demonstrated superior outcomes with primary PTCA compared with fibrinolytic therapy and established primary PTCA as a legitimate alternative and perhaps even preferred reperfusion strategy for patients with acute MI.
Primary Percutaneous Coronary Intervention
Today it is accepted that primary percutaneous coronary intervention (PCI) is the preferred reperfusion strategy for ST-segment elevation MI (STEMI) when it can be performed in a timely manner by experienced personnel.26
Primary Percutaneous Coronary Intervention versus Fibrinolytic Therapy Outcome Comparisons
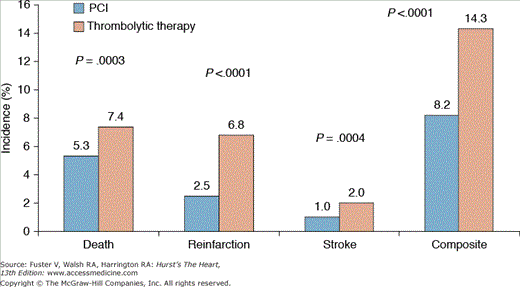
Keeley and Grines performed a meta-analysis of 23 randomized trials incorporating 7739 patients comparing primary PCI with fibrinolytic therapy for STEMI.27 Primary PCI is superior to fibrinolytic therapy in reducing short-term mortality (5.3% vs 7.4%; P = .0003); nonfatal reinfarction (2.5% vs 6.8%; P <.0001); stroke (1.0% vs 2.0%; P = .0004); and the composite end point of death, nonfatal reinfarction, and stroke (8.2% vs 14.3%; P = .0001; Fig. 63–1). These results were maintained at long-term follow-up and were independent of the type of thrombolytic agent used (streptokinase vs fibrin-specific thrombolytics) and whether patients were transferred emergently for primary PCI. The incidence of intracranial hemorrhage was significantly less with primary PCI (0.05% vs 1.1%; P <.0001), but the overall risk of major bleeding (mostly related to access site bleeding) was higher with primary PCI (6.8% vs 5.3%; P = .03).
Figure 63–1.
Meta-analysis of 23 randomized trials comparing short-term outcomes with primary percutaneous coronary intervention (PCI) versus thrombolytic therapy for acute myocardial infarction. Adapted from Keeley et al27 with permission.
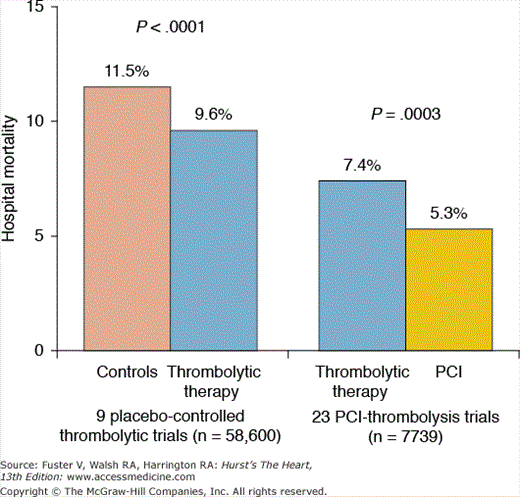
The survival benefit of primary PCI compared with fibrinolytic therapy reported in this meta-analysis was substantial (21 lives saved per 1000 patients treated) and is of similar magnitude to the survival benefit of fibrinolytic therapy compared with placebo reported by the Fibrinolytic Therapy Trialists’ (FTT) Collaborative Group (19 lives saved per 1000 patients treated; Fig. 63–2).28 The relative reduction in death and nonfatal reinfarction is similar across all subgroups of patients treated, including elderly patients, women, diabetics, anterior versus non-anterior infarct location, patients with prior infarction, and patient risk. Of note, the greatest absolute benefit occurs in patients who are at highest risk, especially if they are younger than 75 years of age. The SHOCK (Should We Revascularize Occluded Coronaries for Cardiogenic Shock?) trial, which randomized 302 patients with cardiogenic shock to emergency revascularization versus medical stabilization, found a lower 6-month mortality with emergency revascularization (50% vs 63%; P = .03).29 The survival benefit was especially pronounced in patients who were treated within 6 hours of symptom onset and was seen only in patients younger than age 75 years.
The initial early clinical benefit of primary PCI over fibrinolytic therapy in reducing death and reinfarction appears to be maintained at late follow-up. The PAMI investigators found that death or reinfarction was lower at 2 years with primary PCI than with fibrinolytic therapy (14.9% vs 23.0%; P = .03).30 Similarly, the Zwolle investigators found lower mortality (13.4% vs 23.9%; P = .01) and less reinfarction (6.2% vs 21.9%; P <.0001) with primary PCI than with streptokinase at 5 years.31 Both studies found a lower frequency of hospital readmissions with primary PCI. Keeley’s recent meta-analysis also showed that the initial benefit with primary PCI was maintained at late follow-up at 6 to 18 months.32
If fibrinolysis is the treatment of choice in a clinical scenario, performing angioplasty, or not, should depend on four determining factors: (1) rescue PCI after failed fibrinolysis in patients with persistent symptoms of myocardial injury as demonstrated by lack of ST-segment resolution; (2) immediate PCI (also known as facilitated PCI) regardless of clinical or ECG presence of ischemia; (3) routine delayed PCI in stable patients; and (4) delayed selective and ischemia-driven angioplasty.
The rescue PCI patient subset represents those who have failed fibrinolysis, are at higher risk of mechanical complications, and have higher mortality rates.33 Rescue PCI in the setting of failed fibrinolysis is associated with a 35% reduction in mortality and a 36% reduction in reinfarction. The RESCUE (Randomized Evaluation of Salvage Angioplasty with Combined Utilization of Endpoints) trial investigators randomized 151 patients with first anterior wall MI who were treated with fibrinolytic therapy and who had an occluded infarct artery within 8 hours of symptom onset to rescue PCI versus conservative care.34 The rescue PCI group had a lower composite event rate of death or congestive heart failure and better exercise left ventricular ejection fraction (43% vs 38%; P = .04). These benefits occurred even though stents were not yet available and despite what the authors thought was a strong investigator bias not to randomize patients presenting very early in the course of their infarction. The MERLIN (Middlesbrough Early Revascularization to Limit Infarction) trial investigators randomized patients with STEMI and failed thrombolysis (<50% ST-segment resolution at 60 minutes) to rescue PCI versus conservative care.35 There was no difference in 30-day mortality (the primary end point), but event-free survival was better with rescue PCI (P = .02). The REACT (Rescue Angioplasty Versus Conservative Treatment or Repeat Thrombolysis) trial randomized patients with STEMI and failed fibrinolysis (<50% ST-segment resolution at 90 minutes) to rescue PCI, repeat fibrinolysis, or conservative care.36 Patients treated with rescue PCI had a lower incidence of the composite end point (death, reinfarction, stroke, or heart failure) than did patients treated with either repeat fibrinolytic therapy or conservative care.
Full-dose fibrinolysis followed by early, routine PCI has been considered harmful based on studies performed in the 1980s but more recently has become acceptable in the setting of more potent antiplatelet therapy and coronary stents. This strategy is more beneficial than a conservative approach of ischemia driven-PCI. The PRAGUE (Primary Angioplasty in Patients Transferred From General Community Hospitals to Specialized PTCA Units With our Without Emergency Thrombosis) trial randomized 300 patients to hospitals without PCI facilities to streptokinase, streptokinase, and transfer for immediate PCI or transfer for primary PCI without fibrinolysis.37 Immediate PCI after streptokinase compared with fibrinolysis alone resulted in a trend toward a reduction in the primary 30-day composite end point of death, reinfarction, or stroke (8% vs 15%; P = .12). This trend has been consistent with other observations),38 including a reduction in infarct size by MRI.39 Recently, Cantor et al40 published their observations confirming that routine PCI within 6 hours of tenecteplase therapy is associated with fewer ischemic complications than standard therapy. The high-risk group patients derived the most benefit from early PCI without increased in bleeding.
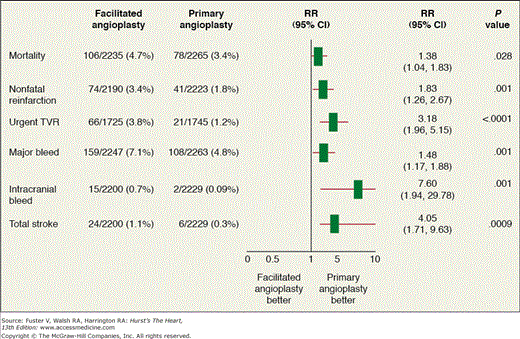
Facilitated PCI includes the administration of full- or reduced-dose fibrinolytic therapy upon presentation followed by transfer for immediate PCI. Although this concept appears appealing and could potentially increase the patency rate in the infarct-related artery (IRA), facilitated PCI is associated with a significant increase in short-term mortality, stroke, reinfarction, and recurrent ischemia necessitating urgent target vessel revascularization (TVR).32 For these reasons, this strategy has fallen out of favor (Fig. 63–3). The additional use of glycoprotein (GP) IIb/IIIa does not appear to confer a significant clinical or angiographic benefit when added to a facilitated PCI strategy as shown in the FINESSE (Facilitated Intervention with Enhanced Reperfusion Speed to Stop Events) trial whereby patients were randomized in a 1:1:1 fashion to receive half-dose reteplase plus abciximab, abciximab alone, or placebo (primary PCI).41 This trial was also prematurely terminated, with 2452 patients (target enrollment, 3000 patients) because of slow recruitment. The largest of these clinical trials the ASSENT (Assessment of the Safety and Efficacy of a New Thrombolytic Regimen)-4 PCI trial42 was terminated prematurely because of a higher in-hospital mortality rate in the facilitated PCI group (6% vs 3%; P = .01).
Figure 63–3.
Pooled analysis of the short-term results from 17 randomized trials comparing facilitated percutaneous coronary intervention (PCI) after fibrinolytic therapy and primary PCI without antecedent pharmacologic therapy in 4504 patients. CI, confidence interval; RR, relative risk; TVR, target vessel revascularization. Adapted with permission from Stone GW. Circulation. 2008;118;552-566.
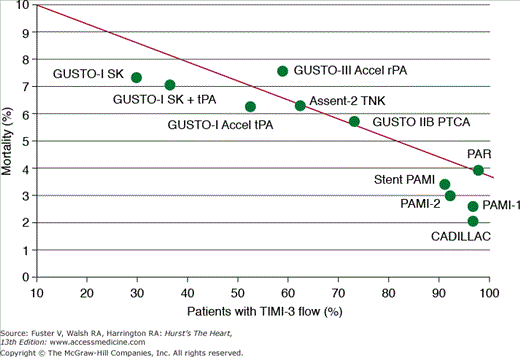
Primary PCI can achieve TIMI-3 flow in the infarct artery in more than 90% of patients43,44 versus 54% with fibrinolytic therapy.45,46 Achieving TIMI-3 flow has a major impact on short- and long-term mortality,45 and the advantage of primary PCI in achieving high rates of TIMI-3 flow is likely responsible for much of the observed mortality advantage. Indeed, there appears to be a tight inverse relationship between the ability to achieve TIMI-3 flow and short-term disability with either reperfusion strategy (Fig. 63–4).43-45,47-52 Newer fibrinolytic strategies using combination therapy with low-dose fibrinolytics and platelet GP IIb/IIIa inhibitors have shown improved TIMI-3 flow rates in small pilot trials,46 but these rates are well below the TIMI-3 flow rates achieved with primary PCI, and they have not shown any mortality advantage in the large GUSTO (Global Utilization of Streptokinase and Tissue Plasminogen Activator for Occluded Arteries) V and ASSENT-3 trials.53,54
Figure 63–4.
Relationship between short-term mortality and the frequency of achieving TIMI (Thrombolysis in Myocardial Infarction)-3 flow measured acutely in the infarct artery in trials with thrombolytic therapy and primary PCI. The thrombolytic trials include GUSTO-I (Global Utilization of Streptokinase and Tissue Plasminogen Activator for Occluded Arteries), GUSTO-III, and ASSENT-2 (Assessment of the Safety and Efficacy of a New Thrombolytic Regimen). The primary percutaneous coronary intervention (PCI) trials include CADILLAC (Controlled Abciximab and Device Investigation to Lower Late Angioplasty Complications), PAMI-1 and -2 (Primary Angioplasty in Myocardial Infarction), Stent PAMI, PAR (Primary Angioplasty Registry) and GUSTO IIB. PTCA, percutaneous transluminal coronary angioplasty; rPA, reteplase; SK, streptokinase; TNK, tenecteplase; tPA, tissue plasminogen activator.
Late angiographic outcomes with primary PCI have been substantially improved with the use of stents.43,55 With stenting, reocclusion occurs in 5% to 6% of patients, restenosis in 20% to 22% of patients, and target vessel revascularization in 7% to 8% of patients. This may further improve with the use of drug-eluting stents (DES; see Stents and Drug-Eluting Stents below). After fibrinolytic therapy, late reocclusion of the infarct artery occurs in 20% to 28% of patients when adjunctive PCI is not used.56,57
Myocardial salvage after primary PCI and fibrinolytic therapy has been evaluated in direct comparisons using paired technetium-99m sestamibi scintigraphy.58-60 These studies showed better myocardial salvage with primary PCI. Most recently, Schomig et al60 found smaller infarct size and better myocardial salvage at all intervals of time to treatment with primary stenting versus fibrinolytic therapy.60 The greater myocardial salvage with primary PCI is probably related to higher frequency of TIMI-3 flow in the infarct artery, less reocclusion, and possibly better myocardial reperfusion.
Adjunctive Therapies with Primary Percutaneous Coronary Intervention
Mechanical reperfusion therapy has improved greatly since its introduction in the early 1980s as a result of increased operator experience, better equipment, and the introduction of new adjunctive therapies. The most important and widely studied of these adjunctive therapies have been platelet inhibitors, stents, distal protection, and thrombectomy.
Five large, randomized trials have evaluated abciximab as adjunctive therapy with primary PCI with STEMI, and all have shown a reduction in the composite end point of death, reinfarction, or urgent TVR at 30 days.43,61-64 These trials have been summarized in a recent meta-analysis.65 The greatest benefit was seen in the ADMIRAL (Abciximab Before Direct Angioplasty and Stenting in Myocardial Infarction Regarding Acute and Long-term Follow-up) trial, in which abciximab was often given early at the time of randomization before primary PCI.62 In addition to better clinical outcomes, the ADMIRAL trial found improved infarct artery patency before PCI and improved left ventricular ejection fraction at 6 months with abciximab. In contrast, The CADILLAC (Controlled Abciximab and Device Investigation to Lower Late Angioplasty Complications) trial showed improvement in 30-day outcomes with abciximab, but most of the benefit of abciximab was seen in patients treated with PTCA alone (4.8 % vs 8.4 %; P =.02) and was without benefit in stented patients (4.5 % vs 5.7 %; P = NS).43 There was no improvement in left ventricular ejection fraction at 7 months. Abciximab did reduce acute thrombosis in stented patients (0 vs 1.0 %; P = .03) as well as in all patients (0.4 % vs 1.4 %; P = .01).
Currently, abciximab has a class IIa indication by American College of Cardiology (ACC)/American Heart Association (AHA) guidelines for use with primary PCI for STEMI,26 and eptifibatide has a IIb recommendation. Because many of the trials showing benefit of abciximab with primary PCI were performed before the routine use of stents and because the largest trial, CADILLAC, showed little benefit in stented patients, the role of GP IIb/IIIa platelet inhibitors with primary PCI remains controversial. Although most trials have not shown an increased incidence of major bleeding with abciximab when used with primary PCI, bleeding is still a major problem when primary PCI is performed with unfractionated heparin (UFH), clopidogrel, and platelet GP IIb/IIIa inhibitors.
Small molecule GP IIb/IIIa platelet receptor inhibitors (eptifibatide and tirofiban) are an attractive option to abciximab in the treatment of patients with STEMI because of the reversibility of the inhibition of platelet aggregation and the lower cost. A recent meta-analysis of all the published data on these agents showed among STEMI patients undergoing primary angioplasty similar results in terms of angiographic, ECG, and clinical outcome.66
The HORIZONS (Harmonizing Outcomes with Revascularization and Stents)–AMI trial compared bivalirudin with UFH plus GP IIb/IIIa inhibition in STEMI patients undergoing primary PCI in an effort to determine the optimum anticoagulant therapy. This trial randomized 3602 patients with STEMI and symptom onset of 12 hours or less. At 30 days, patients who were assigned to receive bivalirudin alone had significantly reduced rate of net adverse clinical events compared with the control group (9.2% vs 12.1%; relative risk [RR], 0.76; 95% confidence interval [CI], 0.63-0.92; P = .005) because of a lower rate of major bleeding (4.9% vs 8.3%; RR, 0.60; 95% CI, 0.46-0.77; P <.001), with similar rates of major cardiovascular events. The clinical benefit of bivalirudin over heparin plus GP IIb/IIIa that is driven by reduced bleeding was preserved at 1 year of follow-up.67
The CLARITY-TIMI-28 (Clopidogrel as Adjunctive Reperfusion Therapy-Thrombolysis in Myocardial Infarction 28) study randomized 3491 patients receiving fibrinolytic therapy within 12 hours of STEMI to clopidogrel (300 mg loading dose plus 75 mg/d oral maintenance dose) or placebo. The primary composite efficacy end point of an occluded infarct related or angiography, death, and recurrent MI before angiography (between 48 and 192 hours after the start of study medication) occurred in 21% of the placebo group and 15% of the clopidogrel group (odds ratio [OR], 0.64; 95% CI, 0.53-0.76) without significant increase in the rate of bleeding.68 Dangas et al69 further demonstrated that pretreatment with 600 mg is superior to 300 mg without an increase in bleeding.
The Stent PAMI trial first documented a clear benefit of stenting by comparing the heparin-coated Palmaz-Schatz stent with balloon angioplasty. Stenting reduced restenosis, reocclusion, and the need for TVR after primary PCI for STEMI.70 However, stented patients had lower TIMI-3 flow rates after PCI and a disturbing trend toward higher mortality at 1 year. The CADILLAC trial evaluated the role of stenting and platelet GP IIb/IIIa inhibition with abciximab in 2082 patients with STEMI who were treated with primary PCI.43 Stented patients had a clear benefit over balloon angioplasty alone in reducing 6-month restenosis (22% vs 41%; P <.01), reocclusion (5.7% vs 11.3%; P = .01), ischemic TVR (6.8% vs 14.7%; P <.001), and major adverse cardiovascular events (MACE) (10.9% vs 18.3%; P <.001). In contrast to Stent PAMI, the CADILLAC trial, using the newer generation MultiLink stent (Guidant Corporation), showed no degradation of TIMI flow after stenting and no difference in 6-month mortality. Based on these data, stenting is recommended for routine use in eligible patients with STEMI who are treated with mechanical intervention.
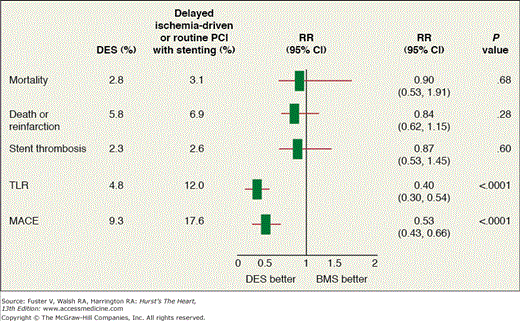
DES have proven very effective in reducing restenosis and TVR compared with bare-metal stents (BMS) after elective and urgent PCI. There is concern that the incidence of stent thrombosis with DES may be higher in the setting of STEMI, and the benefits of DES may not be as great as with elective and urgent PCI.71 Two recent randomized trials found conflicting results with DES versus BMS in STEMI. The TYPHOON (A Multi-center Randomized Trial Comparing Sirolimus-eluting Stents to Bare Metal Stents in Primary Angioplasty for Acute Myocardial Infarction) trial (n = 705) found less TVR with sirolimus-eluting stents versus BMS (5.6% vs 13.4%; P <.001),72 and the PASSION (Randomized Comparison of Paclitaxel-eluting Stent versus Conventional Stent in ST-elevation Myocardial Infarction) trial (n = 617) found no difference in target lesion revascularization between paclitaxel eluting stents and BMS (6.2% vs 7.4%; P = .23).73
In the pooled data from several clinical trials with follow-up ranging from 8 months to 1 year, DES resulted in a marked reduction in target lesion revascularization (TLR) with similar rates of death, reinfarction, and stent thrombosis74 (Fig. 63–5). Consequently, the use of DES is a reasonable approach in patients with STEMI undergoing PCI. Attention must be paid to the issues of long-term dual antiplatelet therapy with aspirin and an adenosine diphosphate (ADP) blocker for all patients receiving a coronary stent, but especially a DES, during primary PCI for STEMI.
Figure 63–5.
Pooled analysis of seven randomized trials comparing drug-eluting stents (DES) and bare-metal stents (BMS) in 2357 total patients with ST-segment elevation myocardial infarction. Follow-up rates are at 12 months in six trials and 8 months in one trial. Mortality data are from five trials (1857 patients). MACE indicates major adverse cardiovascular events consisting of death, reinfarction, or target vessel revascularization, (target lesion revascularization [TLR] in one trial). CI, confidence interval; RR, relative risk. Adapted with permission from Stone GW. Circulation. 2008;118;538-551.
Primary PCI is effective in restoring normal epicardial coronary flow (TIMI-3 flow) in more than 90% of patients with STEMI, but more than half of these patients will have suboptimal flow at the tissue level, as evidenced by poor angiographic blush scores or lack of complete ECG ST-segment resolution.75-77 Although achieving TIMI-3 flow in the epicardial coronary artery is important, optimal outcomes with reperfusion therapy also require optimal myocardial reperfusion. Numerous studies have shown that patients with suboptimal myocardial reperfusion, as evidenced by abnormal myocardial blush or ST-segment resolution, have worse outcomes.75-77
The causes of abnormal myocardial reperfusion after primary PCI are not clear. One potential cause is distal embolization of thrombus and fragments of atherosclerotic plaque at the time of PCI, resulting in obstruction of the distal microcirculation. Several distal protection devices, including the PercuSurge System (Medtronic PercuSurge, Sunnyvale, CA) and the Filter Wire (Boston Scientific, Natick, MA), have been tested in clinical trials with the hope that distal protection will prevent distal embolization and will result in improved myocardial reperfusion and better outcomes. The EMERALD (Enhanced Myocardial Efficacy and Recovery by Aspiration of Liberalized Debris) trial showed no benefit with the Guard-wire (PercuSurge System) in reducing infarct size or improving ST-segment resolution or myocardial blush despite retrieving visible thrombus or atheroma in more than 70 % of patients.44
A number of thrombectomy devices have been used as adjunctive therapy with primary PCI in an attempt to reduce distal embolization, improve microvascular reperfusion, and improve outcomes. Those that have been most thoroughly studied are the X-Sizer Catheter System (EndiCOR Medical, Inc., San Clemente, CA), several aspiration devices, and the AngioJet System (Possis Medical, Minneapolis, MN). A number of small randomized trials have evaluated thrombectomy with primary PCI, and most,78-83 but not all,84,85 have shown improved surrogate end points (ST-segment resolution and myocardial blush, and in some cases, reduced infarct size) with thrombectomy (Tables 63–1 and 63–2).86
| RT | Control | P | |
|---|---|---|---|
| Antoniucci et al (n = 100) | |||
| CTFC | 18 | 23 | 0.03 |
| ST-segment resolution >50% | 90% | 72% | 0.02 |
| Infarct size at 1 mo (sestamibi) | 13.0% | 21.2% | 0.01 |
| AIMI (n = 480) | |||
| TIMI-3 flow | 92% | 97% | 0.02 |
| CTFC | 35 | 32 | 0.13 |
| Grade 2-3 blush | 49% | 58% | 0.06 |
| ST-segment resolution ≥70% | 60 | 68 | 0.14 |
| Infarct size at 14-28 d (sestamibi) | 12.5% | 9.8% | 0.02 |
| AT | Control | P | |
|---|---|---|---|
| REMEDIA trial96 (DIVER) (n = 100) | |||
| Grade 2-3 blush | 63% | 45% | .02 |
| ST-segment resolution ≥70% | 58% | 37% |