Pitavastatin is a potent lipophilic statin and may play an important role in acute myocardial infarction (AMI) but there have been limited data on the safety and efficacy of pitavastatin in AMI. This study consisted of 1,039 consecutive patients with AMI (74.0% men, mean age 61.4 ± 12.6 years) who presented in 10 major percutaneous coronary intervention centers in Korea from February 2007 through September 2009. Pitavastatin 2 mg/day was routinely administered in patients with AMI from time of presentation. We investigated changes of lipid profiles, biochemical markers, adverse events, and clinical outcomes up to 12 months. During the study 318 events overall occurred in 220 patients (21.2%) who reported ≥1 treatment emergent adverse event, although 20 events in 14 patients (1.4%) were treatment-related adverse events. Low-density lipoprotein (LDL) cholesterol percent change was −25.6% and LDL cholesterol target attainment was 70.5% at 12-month follow-up. Levels of creatinine phosphokinase, serum glutamic oxaloacetic transaminase, glutamic pyruvic transaminase, and high-sensitivity C-reactive protein decreased significantly during the first 1 month of pitavastatin treatment and were sustained to 12-month follow-up. Major adverse cardiac events occurred in 66 patients (7.3%). All-cause deaths occurred in 32 patients (3.5%) including 19 (2.1%) cardiac deaths and recurrent MIs occurred in 14 (1.6%) and target lesion revascularizations in 42 (4.7%). In conclusion, administration of pitavastatin 2 mg/day in patients with AMI showed 70.5% LDL cholesterol target attainment with good tolerance and was associated with favorable clinical outcomes up to 12 months.
Pitavastatin (Livalo), a potent inhibitor of 3-hydroxy-3-methyl-glutaryl-coenzyme A reductase, was launched in Japan in 2003 and currently is commonly used in Asia. In the present study we sought to evaluate the long-term efficacy and safety and clinical outcomes after routine administration of pitavastatin in patients with acute myocardial infarction (AMI) as a substudy of the Korea Acute Myocardial Infarction Registry (KAMIR).
Methods
The Pitavastatin ( L ivalo) Acute Myocardial Infarction Study (LAMIS) is a substudy of the KAMIR. LAMIS investigators were selected from 10 major percutaneous coronary intervention (PCI) centers among the 50 KAMIR sites and 1,039 consecutive patients with AMI who received pitavastatin as the sole statin were prospectively enrolled from February 2007 through September 2009. The KAMIR is a Korean, prospective, open, observational, multicenter online registry of AMI with support from the Korean Society of Cardiology. These participating hospitals can provide primary PCI. Details of the KAMIR have been published. AMI was diagnosed by characteristic clinical presentation, serial changes on electrocardiogram suggesting infarction, and increase in cardiac enzymes. ST-segment elevation MI was defined by new ST-segment elevation in 2 contiguous leads measuring 0.2 mV in leads V 1 to V 3 or 0.1 mV in all other leads. We enrolled patients if they had an AMI including ST-segment elevation MI and non–ST-segment elevation MI. Diagnostic angiography and PCI were performed after premedication with aspirin (≥100 mg) and unfractionated heparin or low-molecular-weight heparin. Loading of aspirin (200 to 300 mg) and clopidogrel (300 to 600 mg) was performed before PCI. Addition of cilostazol as a triple antiplatelet and/or glycoprotein IIb/IIIa receptor inhibitor was left to the physician’s discretion. Coronary angiography was performed through the femoral or radial artery. Unfractionated heparin was infused throughout the procedure to maintain an activated clotting time of ≥250 seconds. Stents were deployed after balloon angioplasty and use of a drug-eluting stent was left to the physician’s discretion. We prescribed aspirin 100 mg/day indefinitely and clopidogrel 75 mg/day for ≥6 months. Pitavastatin 2 mg/day was routinely administered in patients with AMI from time of presentation. The protocol was approved by the institutional review board or ethics committee at each participating centers.
Total cholesterol, triglycerides, low-density lipoprotein (LDL) cholesterol, high-density lipoprotein (HDL) cholesterol, and high-sensitive C-reactive protein were measured. LDL cholesterol target attainment by the National Cholesterol Education Program/Adult Treatment Panel III guideline was also evaluated at 12 months. The primary study end point was all-cause mortality at 12 months after the index PCI. The secondary study end points included individual hard end points and cumulative major adverse cardiac events (MACEs) at 12 months. Total MACEs were defined as the composite of (1) total death, (2) nonfatal MI, and (3) repeated PCI or coronary artery bypass grafting. All deaths were considered cardiac deaths unless a noncardiac death could be excluded. Recurrent MI was defined as the development of pathologic Q wave in ≥2 contiguous leads or an increase in creatine kinase level to >2 times the upper limit of normal with an increase of creatine kinase-MB isoenzyme. Target lesion revascularization was defined as ischemia-driven PCI of a target lesion because of restenosis or reocclusion within the stent or in the adjacent 5 mm of the distal or proximal segment.
Safety assessments consisted of monitoring and recording treatment emergent adverse events, blood chemistry, and physical examination including vital signs. The investigator decided whether each adverse event was related to treatment with pitavastatin and decided whether any changes were clinically important and related to pitavastatin. Levels of creatinine phosphokinase, serum glutamic oxaloacetic transaminase, and serum glutamic pyruvic transaminase were evaluated at baseline, 1 month, 6 months, and 12 months.
Data are expressed as mean ± SD and frequencies are expressed as percentages. Differences between measurements were tested using t test for continuous variables and chi-square test for categorical variables. A probability value <0.05 was considered statistically significant. Multivariate logistic regression analysis was performed to determine the independent predictor for MACEs. The model included all variables with a p value <0.1 in univariate analysis. All statistical analyses were performed using SPSS 17.0 (SPSS, Inc., Chicago, Illinois).
Results
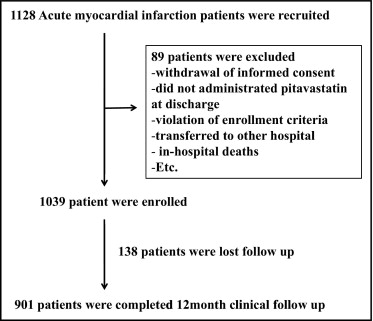
During the study period 1,128 patients were recruited, but 89 patients were excluded because of withdrawal of informed consent, violation of enrollment criteria, transfer to another hospital, and in-hospital deaths ( Figure 1 ) . Therefore 1,039 patients were enrolled for this study. Baseline characteristics of the overall study population are presented in Table 1 . Mean age was 61.4 ± 12.6 years, 74.0% were men, mean body mass index was 24.2 ± 3.2 kg/m 2 , 46.3% had hypertension, 24.2% had diabetes, 11.0% had a history of coronary artery disease, 9.8% had dyslipidemia, 4.9% previously used a statin, and 48.3% were current smokers. Of 1,039 patients with AMI 660 (63.8%) presented with ST-segment elevation MI and 781 patients (75.6%) were admitted within 12 hours onset of AMI ( Table 1 ). As the initial therapeutic strategy for ST-segment elevation MI 532 patients underwent primary PCI, 45 patients underwent facilitating PCI, 37 patients received thrombolytics, and 35 patients were managed by conservative treatment. Of 375 patients (36.2%) with non–ST-segment elevation MI 244 patients received early invasive therapy and the other 124 patients received early conservative therapy.
| Characteristics | Pitavastatin (n = 1,039) |
|---|---|
| Age (years) | 61.4 ± 12.6 |
| Men | 769 (74.0%) |
| Body mass index (kg/m 2 ) | 24.2 ± 3.2 |
| Diabetes mellitus | 251 (24.2%) |
| Hypertension | 480 (46.3%) |
| Dyslipidemia ⁎ | 102 (9.8%) |
| Statin use | 50 (4.9%) |
| Previous coronary artery disease † | 114 (11.0%) |
| Current smoker | 499 (48.3%) |
| Systolic blood pressure (mm Hg) | 116.6 ± 19.4 |
| Diastolic blood pressure (mm Hg) | 71.7 ± 12.4 |
| Heart rate (beats/min) | 74.5 ± 14.3 |
| Total cholesterol (mg/dl) | 190.9 ± 42.4 |
| Triglyceride (mg/dl) | 125.2 ± 91.4 |
| High-density lipoprotein cholesterol (mg/dl) | 45.2 ± 11.8 |
| Low-density lipoprotein cholesterol (mg/dl) | 122.1 ± 37.1 |
| High-sensitive C-reactive protein (mg/L) | 10.0 ± 29.8 |
| Creatinine phosphokinase, maximum (IU/L) | 1,129.5 ± 2,135.0 |
| Type of acute myocardial infarction | |
| ST-segment elevation myocardial infarction | 660 (63.8%) |
| Non–ST-segment elevation myocardial infarction | 375 (36.2%) |
| Killip classification | |
| I | 817 (80.0%) |
| II | 154 (15.1%) |
| III | 34 (3.3%) |
| IV | 16 (1.6%) |
| Onset time of acute myocardial infarction | |
| Within 12 hours | 781 (75.6%) |
| Within 24 hours | 93 (9.0%) |
| Within 48 hours | 44 (4.3%) |
| After 48 hours | 115 (11.1%) |
| Number of coronary artery narrowed | 1,022 |
| 0 | 26 (2.5%) |
| 1 | 470 (46.0%) |
| 2 | 341 (33.4%) |
| 3 | 185 (18.1%) |
| Angiographic characteristics | |
| Reference vessel diameter (mm) | 3.1 ± 0.6 |
| Minimal luminal diameter (mm) | 0.38 ± 0.45 |
| Lesion length (mm) | 23.2 ± 6.2 |
| Stent size (mm) | 3.2 ± 0.5 |
| Drug-eluting stent | 98.8% |
| Bare-metal stent | 1.2% |
| Treatment of acute myocardial infarction | 1,014 |
| Percutaneous coronary intervention | 959 (94.6%) |
| Thrombolysis | 51 (5.0%) |
| Coronary bypass | 4 (0.4%) |
| Stage of revascularization | 982 |
| No revascularization | 58 (5.9%) |
| Revascularization of single infarct-related artery | 511 (52.0%) |
| Revascularization of only infarct-related artery in multivessel disease | 153 (15.6%) |
| Multivessel coronary revascularization | 121 (12.3%) |
| Total coronary revascularization | 139 (14.2%) |
⁎ Diagnosis previously made by physician or receiving lipid-lowering therapy.
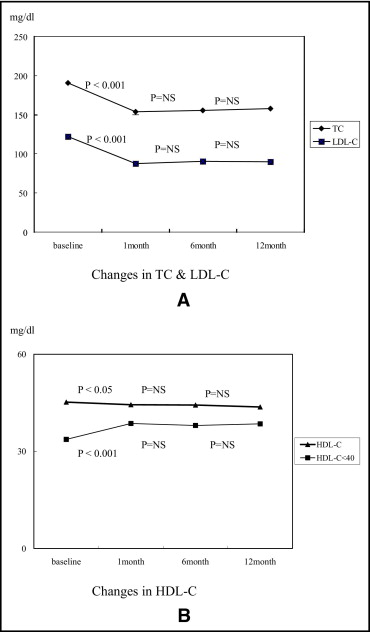
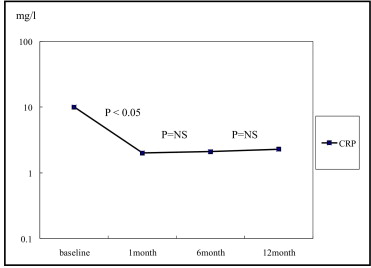
Not all efficacy data were always available during the study period. Thus lipid changes were analyzed in patients whose data were available at 12 months. Of the total 1,039 patients 340 patients had data available for total cholesterol, 319 for triglycerides, 318 for HDL cholesterol, and 319 for LDL cholesterol. Baseline mean total cholesterol, triglyceride, HDL cholesterol, and LDL cholesterol levels were 191 ± 42, 125 ± 91, 45 ± 12, and 122 ± 37 mg/dl, respectively. At 12 months mean total cholesterol, triglyceride, HDL cholesterol, and LDL cholesterol levels were 158 ± 35.0, 151 ± 152, 44 ± 10, and 90 ± 29 mg/dl, respectively ( Table 2 ). We found that total cholesterol and LDL cholesterol were decreased significantly during the first 1 month of pitavastatin treatment (total cholesterol 191 ± 42 vs 154 ± 29 mg/dl, p <0.001; LDL cholesterol 122 ± 37 vs 88 ± 25 mg/dl, p <0.001) and sustained throughout the 12-month follow-up ( Figure 2 ) . Mean HDL cholesterol was decreased 2.4% from baseline to 12 months (45 ± 12 vs 44 ± 10 mg/dl, p <0.05) in all patients. Interestingly, for the low HDL group (HDL cholesterol <40 mg/dl, n = 99 patients) HDL cholesterol was increased by 13.5% (34 ± 4 vs 39 ± 8 mg/dl, p <0.001; Figure 2 ). Percent changes of mean total cholesterol, mean LDL cholesterol, and mean HDL cholesterol showed decreases of 16.7%, 25.6%, and 2.4%, respectively, in all patients. In addition, 70.5% of patients had achieved the LDL cholesterol target defined by National Cholesterol Education Program criteria and LDL cholesterol target attainment for diabetic patients was 67.2% ( Table 3 ). The inflammatory marker high-sensitive C-reactive protein was remarkably high at baseline but normalized during the first 1 month of pitavastatin treatment and was sustained up to 12-month follow-up (10.0 ± 29.8 vs 2.3 ± 13.1 mg/dl, p <0.05; Figure 3 ) .
| Before Discharge | 1 Month | 6 Months | 12 Months | |
|---|---|---|---|---|
| Total cholesterol (mg/dl) | 191 ± 42 | 154 ± 29 | 156 ± 34 | 158 ± 35 |
| Triglyceride (mg/dl) | 125 ± 91 | 145 ± 108 | 139 ± 77 | 151 ± 152 |
| High-density lipoprotein cholesterol (mg/dl) | 45 ± 12 | 44 ± 11 | 44 ± 11 | 44 ± 10 |
| Low-density lipoprotein cholesterol (mg/dl) | 122 ± 37 | 88 ± 25 | 91 ± 27 | 90 ± 28 |
| Creatinine phosphokinase (IU/L) | 1,130 ± 2,135 | 105 ± 95 | 120 ± 97 | 117 ± 79 |
| Glutamic oxaloacetic transaminase (IU/L) | 91 ± 139 | 25.1 ± 17.4 | 26 ± 26 | 25 ± 10 |
| Glutamic pyruvic transaminase (IU/L) | 40 ± 44 | 28 ± 26 | 27 ± 29 | 26 ± 16 |
| High-sensitive C-reactive protein (mg/L) | 10.0 ± 29.8 | 2.0 ± 6.8 | 2.1 ± 9.4 | 2.3 ± 13.1 |
| Before Discharge | 1 Month | 6 Months | 12 Months | |
|---|---|---|---|---|
| (n = 1,007) | (n = 540) | (n = 438) | (n = 319) | |
| Low-density lipoprotein attainment | 274 (27.2%) | 378 (70.0%) | 293 (66.9%) | 225 (70.5%) |
| Diabetic patients | 78 (31.7%) | 96 (74.4%) | 62 (69.7%) | 45 (67.2%) |
| Nondiabetic patients | 196 (25.9%) | 281 (68.7%) | 231 (66.6%) | 180 (71.7%) |
In total 1,039 patients were evaluated for safety throughout the follow-up period. Overall 318 events (30.6%) in 220 patients (21.2%) were reported including ≥1 treatment emergent adverse event and 20 events in 14 patients were treatment-related adverse events. Investigators considered that 15 events (4.7%) were related to pitavastatin and 5 events (1.6%) were probably related to pitavastatin treatment ( Table 4 ). Furthermore, 11 events caused a hold of pitavastatin treatment and 32 events ended in withdrawal from the study. Most treatment emergent adverse events (276 of 318, 86.8%) were classified as not serious, whereas 42 of 318 events (13.2%) were classified as serious treatment emergent adverse events. We also found that mean creatinine phosphokinase, glutamic oxaloacetic transaminase, and glutamic pyruvic transaminase levels normalized during the first 1 month of pitavastatin treatment and was sustained up to 12-month follow-up ( Table 2 ). Common adverse treatment-related adverse events were 4 cases of serum glutamic oxaloacetic transaminase/glutamic pyruvic transaminase increase, 3 cases of myalgia, and 1 case of creatinine phosphokinase increase. There were no reports of myopathy, myositis, or rhabdomyolysis throughout the 12-month follow-up period.



