Left Ventricular Reconstruction
Lorenzo Menicanti
Serenella Castelvecchio
OUTLINE
The increase in left ventricular (LV) volume after a myocardial infarction (MI) is a component of the remodeling process and it is associated with a poor clinical outcome. Hence, the current management strategy for ischemic LV dysfunction has been aimed to reverse the remodeling process (i.e., reduction of LV volume and improved ejection fraction [EF]) by medical therapy, devices, and/or surgical strategies. Surgical left ventricular reconstruction (LVR) has been introduced as an optional therapeutic strategy aimed to reduce LV volumes through the exclusion of the scar tissue, thereby restoring the physiological volume and shape and improving LV function and clinical status. Later, the technique has been widely adopted by skilled surgeons and refined in an effort to standardize the procedure and to optimize the results. Until recently, several studies have shown that surgical LVR is effective and relatively safe with a favorable 5-year outcome. However, in spite of the large amount of reports drawn on various data sets, the additional benefit of LVR to CABG remains debated.
This chapter briefly discusses the rationale to surgically reverse LV remodeling through LVR, and, more extensively, the technique and the indications to the best of our knowledge.
INTRODUCTION
Despite many breakthroughs in cardiovascular medicine, MI and heart failure (HF) are still among the most major public health challenges in the developed countries. The 5-year survival rate of patients diagnosed with HF is still <50% and might even be underestimated. HF is associated with ischemic heart disease in a percentage of patients ranging from 46% to 68%. Research has been very effective in delivering major advances in therapy of ischemic HF patients, including drugs, device therapy, and surgery. However, despite advances in different therapeutic strategies, the prognosis for patients with chronic ischemic HF remains poor. Indeed, HF is a syndrome with a broad spectrum of heterogeneous symptoms and signs caused by cardiac dysfunction and resulting in a wide range of clinical expressions. Treating generically, HF syndrome is reductive and misleading: to be really successful, the underlying disease, named LV remodeling, should be addressed and treated.
LEFT VENTRICULAR REMODELING: MECHANISMS AND CHARACTERISTICS
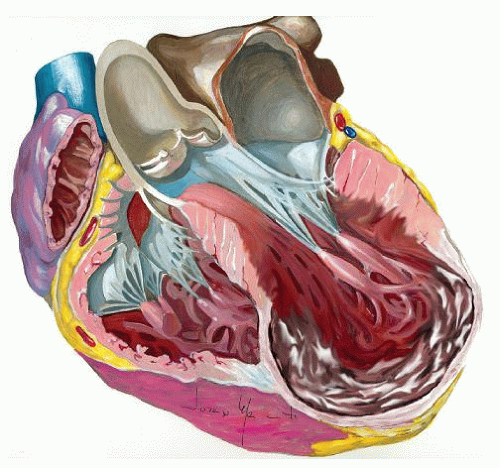
LV remodeling is a complex, dynamic, and time-dependent process that may occur in different clinical conditions, including MI, leading to chamber dilatation, altered configuration, and increased wall stress. MI, particularly large, transmural infarctions, results in a complex of structural changes involving both the infarcted and noninfarcted zones. LV remodeling usually begins within the first few hours after an MI and results from fibrotic repair of the necrotic area with scar formation, elongation, and thinning of the infarcted zone. LV volumes increase, a response that is sometimes considered adaptive, associated with stroke volume augmentation in an effort to maintain a normal cardiac output as the EF declines. However, beyond this early stage, the remodeling process is driven predominantly by eccentric hypertrophy of the noninfarcted remote regions, resulting in increased wall mass, chamber enlargement, and geometric distortion (Fig. 54.1). These changes, along with a decline in performance of hypertrophied myocyte, increased neurohormonal activation, collagen synthesis, fibrosis, and remodeling of the extracellular matrix within the noninfarcted zone, result in a progressive decline in ventricular performance. Left untreated, LV hypertrophy, dilatation, and contractile dysfunction may progress indefinitely as evidenced by progressive increases in LV volumes.
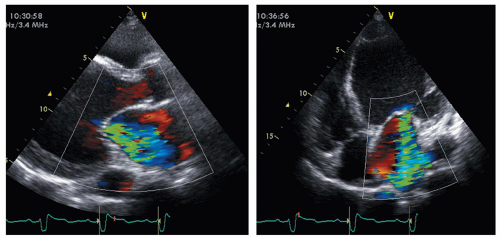
Furthermore, as part the complex process of LV remodeling, functional mitral regurgitation (MR) may occur to adversely affect the prognosis (Fig. 54.2). Chronic ischemic MR occurs in approximately 20% to 25% of patients followed up after MI and in 50% of those with postinfarct congestive HF. The papillary muscle displacement, which may occur as a consequence of the LV dilatation, results in tenting of the mitral valve at closure with lack of a proper coaptation, in turn leading to secondary MR. In addition, ventricular dilatation results in annular enlargement, which further increases valve incompetence. It is well known that functional MR, causing LV volume overload, worsens prognosis. Until recently, it was unclear if the volume overload created by MR adds a greater pathologic burden to an already adverse condition or, simply, the worse prognosis is related to a poorer LV function, and functional MR is merely an indicator of this bad condition. To this regard, a major contribution has recently been provided by Rossi and coworkers in a large population of patients affected by dilated cardiomyopathy and HF. The authors found that functional MR was strongly associated with the outcome after adjustment of left ventricular ejection fraction (LVEF) and restrictive mitral filling pattern, either in patients with ischemic dilated cardiomyopathy or nonischemic.
The Rationale to Perform Left Ventricular Reconstruction in Reversing Left Ventricular Remodeling
Surgical LVR has been introduced as an optional therapeutic strategy aimed to reduce LV volumes through the exclusion of the scar tissue, thereby reducing the ventricle to a more physiological volume, reshaping the distorted chamber, and improving cardiac function through a reduction of LV wall stress in accordance with the principle of Laplace’s law. Since LV wall stress is directly proportional to LV internal radius and pressure and inversely proportional to wall thickness, any intervention to optimize this relationship would be beneficial either in terms of improving wall compliance and reducing filling pressure or, as wall stress is a crucial determinant of afterload, in terms of enhancing contractile performance of LV by increasing the extent and velocity of systolic fiber shortening. Furthermore, LVR of failing ventricles is usually combined with myocardial revascularization with the aim to treat the underlying coronary artery disease. Finally, although the matter of functional chronic ischemic MR, in terms of whether, when, and how it should be corrected is still considerably controversial, it should be pointed out that LVR offers either the possibility to repair the mitral valve through the LV opening or the potential of improving mitral functioning by reducing LV volumes, papillary muscles distance (which is a main determinant of functional MR), and hence rebuilding a more normal geometry.
Left Ventricular Reconstruction Technique
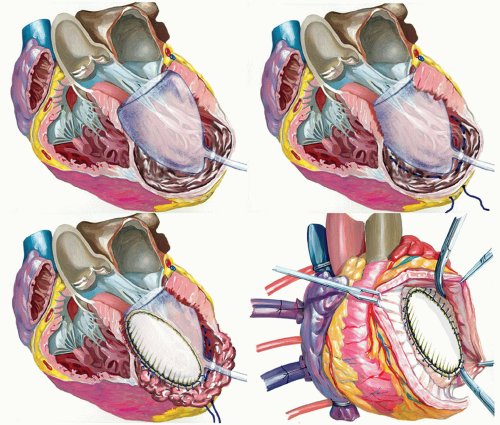
After the first description of the linear suture by Cooley in 1958 and the circular external suture described by Jatène in 1984, Dor started to use a circular patch to reconstruct LV cavity (“endoventricular circular patchplasty”—EVCPP), addressing anatomic situations different from the classical LV aneurysm. The technique, performed under total cardiac arrest, involved the opening of the ventricle in the center of the depressed area, thrombectomy when indicated, and exclusion of dyskinetic or akinetic LV free wall through an endoventricular circular suture passed in the tissue above the transitional zone. A Dacron patch was secured at the junction of the endocardial muscle and scarred tissue, thereby excluding noncontractile portions of the LV and septum. In 1998, Dor further refined the technique by placing a volume-measuring device, in the form of a saline-filled balloon, into the ventricle before tightening the suture, thereby standardizing the conduct of the operation and ensuring that the ventricle was left neither too large nor too small. Later, the procedure has been adopted by many surgeons leaving, however, the technique far from a real standardization and making the results difficult to compare. McCarthy described a double purse-string suture nopatch technique. Mickleborough described a tailored scar excision along with septoplasty, when indicated (dyskinetic septum), and modified linear closure. We adopted a technique that does not differ substantially from the Dor procedure except for the use, since July 2001, of a preshaped mannequin (TRISVRTM, Chase Medical Inc., Richardson, TX; Fig. 54.3). The operation is performed under total cardiac arrest, with antegrade cold blood cardioplegia. According to the potential of this surgery to address “ischemia, ventricle and valve,” we perform the procedure as follows.
Ischemia
Complete myocardial revascularization is performed first with particular attention to revascularize the proximal left anterior descending segment, to preserve the
upper part of the septum. For this purpose, a left internal mammary artery is almost always placed on the left anterior descending artery. Revascularization is completed, when indicated, through sequential saphenous vein coronary bypass grafting on right and circumflex arteries.
upper part of the septum. For this purpose, a left internal mammary artery is almost always placed on the left anterior descending artery. Revascularization is completed, when indicated, through sequential saphenous vein coronary bypass grafting on right and circumflex arteries.
Ventricle
After completion of coronary grafting, the ventricle is opened with an incision parallel to the left anterior descending artery, starting at the middle scarred region and ending at the apex. The cavity is carefully inspected and any thrombus is removed if present. The surgeon must be careful to identify the transitional zone between scarred and nonscarred tissue, driven by cardiac magnetic resonance (CMR) with late gadolinium enhancement (LGE), when previously performed, or alternatively by echocardiographic analysis. After that, a preshaped mannequin is inserted into the LV chamber and inflated with saline (Fig. 54.3, upper panel on the left). The mannequin is useful to optimize the size and shape of the new LV, particularly when the ventricle is not very enlarged (to reduce the risk of a too small residual cavity), or when the transitional zone between scarred and nonscarred tissue is not clearly demarcated, as occurs in akinetic aneurysms. Furthermore, the mannequin is useful in giving the surgeon the correct position of the apex and in maintaining the long axis of the ventricle in a physiologic range (7.5/8.5), reducing thereby the risk of sphericalization of the new ventricle. The size of the device is defined by multiplying the body surface area of the patient by 50 or 60 ml. The exclusion of dyskinetic or akinetic LV free wall is performed through an endoventricular circular suture passed in the tissue of the transitional zone (Fig. 54.3, upper panel on the right). The conical shape of the mannequin guides the orientation of the plane of the endoventricular circular suture at the transitional zone, obliquely toward the aortic flow tract, mainly in rebuilding the new apex. The reconstruction of the apex may be difficult when the apical and inferior regions are severely dilated and scarred; in this case, we apply a modification of the Dor procedure that involves plication of the distal inferior wall before patch placement, thus placing the apex in a more superior position and leaving a small portion of scar (Fig. 54.4). The mannequin is removed before the closure of the ventricle and the opening is closed with a direct suture (simple stitches tangential to the mannequin) if it is <3 cm large or with an elliptical, synthetic patch if >3 cm to avoid distortion of the cavity (Fig. 54.3, lower panel on the left). In the first case, a second stratum with the excluded tissue is sutured on the first suture to avoid bleeding. If the closure is performed by using a patch, a few millimeters of border is left sewing the patch in the everting way (Fig. 54.3, lower panel on the right). This technique assures a good hemostasis and makes easier to
put some stitches, if needed. The positioning of the patch, which follows in some way the endoventricular circular suture, is crucial in determining the residual shape of the new ventricle. To this aim, we pay attention to positioning the patch with an oblique orientation, toward the aortic outflow tract. In this way, we avoid to obtain a box-like shape of the ventricle that may occur when the orientation of the patch is almost parallel to the mitral valve. Usually, a Dacron patch is used; the pericardial patch has been abandoned because of the risk of heavy calcification within the heart. More recently, research is focused on the use of a patch that should be able to promote an ingrowing of stem cells; however, data are not available yet.
put some stitches, if needed. The positioning of the patch, which follows in some way the endoventricular circular suture, is crucial in determining the residual shape of the new ventricle. To this aim, we pay attention to positioning the patch with an oblique orientation, toward the aortic outflow tract. In this way, we avoid to obtain a box-like shape of the ventricle that may occur when the orientation of the patch is almost parallel to the mitral valve. Usually, a Dacron patch is used; the pericardial patch has been abandoned because of the risk of heavy calcification within the heart. More recently, research is focused on the use of a patch that should be able to promote an ingrowing of stem cells; however, data are not available yet.
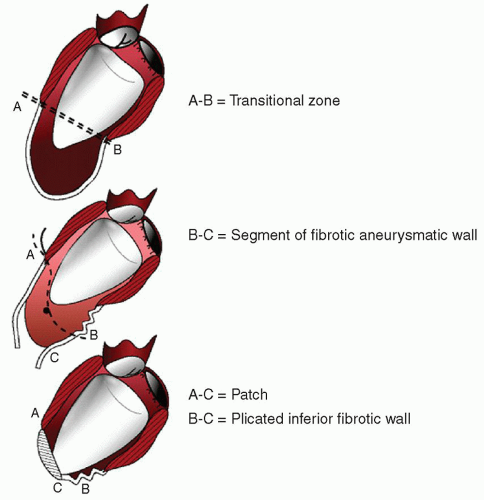 Fig. 54.4. The plication at the inferolateral portion of the ventricle is useful to lift up the new apex. |
Valve
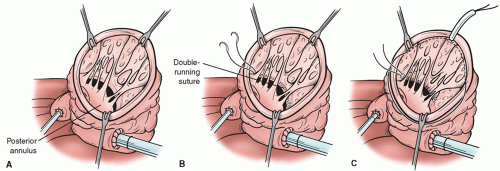
When indicated, mitral valve is repaired through the ventricular opening with a double-arm stitch running from one trigone to the other one, embedding the two arms in the posterior annulus of the mitral valve (Fig. 54.5). To avoid tears of the posterior left of the mitral valve, we reinforce the suture with a Teflon strip. After that, the suture is tied to undersize the mitral orifice. A Hegar sizer No. 26 is used to trim the mitral annulus. Alternatively, a restrictive mitral annuloplasty with a ring implantation has been recently introduced for selected patients, when the LV opening is not big enough to have a good exposition of the mitral valve.
Special Conditions
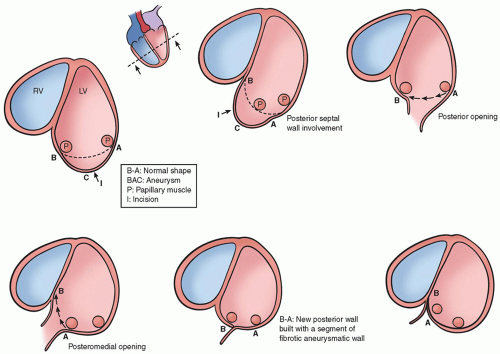
The surgical procedure as described above is usually performed to reverse LV remodeling after an anterior MI. However, beside the LV remodeling following an anterior MI, other pattern of postinfarction LV remodeling may occur, according to the site of coronary occlusion and varying from the classic posterior aneurysm with a bulging of the inferior wall and good contraction of the remaining cavity to a global dilatation of the LV chamber with regional wall dysfunction at the inferior and posterobasal region. In these situations, the same concept used for anteroseptal dilatation can be applied, excluding the scar tissue and reducing the volume. Surgery for the posterior aneurysm generally involves a patch to close the neck of dilatation. However, the treatment of global dilatation of the inferoposterior wall is more complex, especially for the relationship between the scar and the dilatation with respect to the papillary muscles. After an inferior MI,
there are two possibilities: (a) the dilatation is mainly between the two papillary muscles or (b) the dilatation is between the posteromedial papillary muscle and the septum, which is deeply involved. We use two techniques for LV dilatation after an inferior MI. The first (Fig. 54.6, on the left panel) involves the opening of the scarred wall at the level of the scar or at the level of the collapsed area, parallel to the posterior descending artery. A continuous 2-0 Prolene suture is performed to obtain the reapproximation of the two papillary muscles and the exclusion of the entire dilated zone. The suture is started at the beginning of the dilatation (sometimes just at the level of the mitral annulus) and continues toward the apex. According to the second technique (Fig. 54.6, on the right panel), the wall is opened and the continuous suture is brought behind the posteromedial papillary muscle, bringing the posterior wall against the septum.
there are two possibilities: (a) the dilatation is mainly between the two papillary muscles or (b) the dilatation is between the posteromedial papillary muscle and the septum, which is deeply involved. We use two techniques for LV dilatation after an inferior MI. The first (Fig. 54.6, on the left panel) involves the opening of the scarred wall at the level of the scar or at the level of the collapsed area, parallel to the posterior descending artery. A continuous 2-0 Prolene suture is performed to obtain the reapproximation of the two papillary muscles and the exclusion of the entire dilated zone. The suture is started at the beginning of the dilatation (sometimes just at the level of the mitral annulus) and continues toward the apex. According to the second technique (Fig. 54.6, on the right panel), the wall is opened and the continuous suture is brought behind the posteromedial papillary muscle, bringing the posterior wall against the septum.
DRAWBACKS AND SUGGESTIONS
Inside the Ventricle
Despite the use of the sizing device, the residual volume is not always congruent with the expected volume, since the operation depends not only on the preoperative volume but also on the preoperative shape, on the papillary muscles size and position, on the presence of trabeculae, or on the LV compliance. Despite any efforts to describe different LV shape abnormalities (Type 1, Type 2, and Type 3, according to Di Donato’s classification, or using old or new indexes, including sphericity index, eccentricity index, and/or conicity index), the true anatomy of the ventricle and the extension of the damage might not be extensively clear before the opening of the cavity. This is especially true when the anterior septum is deeply involved (Fig. 54.7). In this case, noninvasive imaging testing is mandatory to evaluate the feasibility of the procedure and to tailor eventually the operation for a specific patient.
Regarding the impact of LVR on LV compliance, our group reported for the first time that the likelihood for diastolic function worsening after LVR is higher when the LV cavity is globally dilated (i.e., akinetic aneurysm) and relatively too small




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree