Infective Endocarditis: Introduction
Infective endocarditis (IE) is a disease caused by microbial infection of the endothelial lining of intracardiac structures and is invariably fatal if untreated. Infection most commonly resides on one or more heart valve leaflets, but may involve mural endocardium, chordal structures, myocardium, and pericardium. The presence of an intracardiac or endovascular device provides a nidus for infection, as well as a barrier to eradication. Despite significant advances in the diagnosis and treatment of IE, 6-month mortality rates still approach 25%.1,2 Changes in both patient demographics and microbial biology have challenged conventional wisdom. Prompt recognition, triggered by a high index of clinical suspicion in susceptible patients, early diagnosis, and aggressive treatment, are the critical components of a successful management strategy. Combined medical and surgical interventions can lead to improved outcomes for selected patients. Patient education, attention to general oral-mucosal hygiene, and the appropriate use of prophylactic antibiotics are the mainstays of prevention.
History
The earliest description of the vegetative lesion of IE has been attributed to Lazarus Riverius (1589-1655).3 During his tenure as Vatican physician to Pope Clement XI, Giovanni Lancisi (1654-1720) recorded additional observations in De Subitaneis Mortibus (1709).4 Despite these descriptions, it was not until the mid-19th century that a connection was made between vegetative lesions, systemic inflammation (Boulllard, 1841), and embolic phenomena (Virchow, 1847; Kirkes, 1852).5 Sir William Osler made several important advances in the understanding of IE, as summarized in his famed Gulstonian lectures of 1885.6 He defined IE as a primary “mycotic” process and provided the first formal description of two clinical variants of the disease—an acute and fulminating form versus a chronic and insidious form. Despite his extensive knowledge of the disease, Osler was also the first to acknowledge the reality of diagnostic uncertainty in many cases.
Epidemiology
In the first half of the 20th century, IE was predominantly a complication of rheumatic heart disease and poor dentition. In developing countries, rheumatic heart disease remains the most frequent predisposing cardiac condition.7 However, the epidemiologic features of IE in developed countries have changed considerably. The aging of the population has been paralleled by increases in the prevalence of degenerative heart valve disease and in the use of implanted heart valve substitutes and intracardiac devices. The numbers of patients with chronic, predisposing medical comorbidities, such as diabetes, HIV infection, and end-stage renal disease, have also increased, as has the commensurate risk of exposure to nosocomial bacteremia, often with antibiotic resistance.8-10 A recent prospective study of 2781 adults with IE found that 25% of cases were associated with recent health care exposure.2 These changing demographics are reflected in two observations: First, the median age of patients with IE has gradually increased from 30 to 40 years in the pre-antibiotic era to 47 to 69 years in the late 20th century.2,11,12 Second, the incidence of IE in developed countries has remained unchanged, despite the dramatic reduction in the incidence of rheumatic heart disease over the last half century.
The incidence of IE has been difficult to estimate due principally to the challenges of accurate case definition and identification of representative populations at risk (eg, urban vs rural, injection drug users, young vs elderly). Over the last 15 years, several well-designed epidemiologic studies have attempted to address these problems9,11,13-17 and are summarized in Table 86–1. A number of these studies suggest that the incidence of IE is increasing among the elderly.9,11,16,17 Increased patient longevity and the explosive use of health care interventions predict that the incidence of IE throughout the world will continue to rise.18
| Study | Population Studied | IE Incidence (cases per 100,000 person-years) | Comments |
|---|---|---|---|
| Hogevik et al11 | Well-defined urban population in Goteborg, Sweden, followed prospectively from 1994-1998. | 5.9 | IE incidence was age- and sex-adjusted. Crude incidence was 6.2. In the oldest age group (80-89 years), the annual incidence was 22. |
| Berlin et al13 | Retrospective analysis of IE cases in metropolitan Philadelphia from 1988-1990. | 11.6 | The population studied had a high proportion of injection drug users, a population whose rate of IE was 5.2 cases/100,000 person-years. |
| Delahaye et al14 | Retrospective analysis of 415 IE cases collected from a combined urban and rural population from 3 regions in France in 1991. | 2.4 | IE incidence was age- and sex-adjusted. The study population had only 5% injection drug users. |
| Tleyjeh et al15 | Retrospective analysis of 107 IE cases occurring in Olmsted County, MN, between 1970 and 2000. | 5.0-7.0 | IE incidence was age- and sex-adjusted. Increasing temporal trend was observed for PVE and for cases associated with mitral valve prolapse. |
| Hoen et al9 | Retrospective analysis of 390 IE cases in 6 regions of France in 1999. | 3.1 | IE incidence was age- and sex-adjusted. There was a high incidence of IE in the elderly of 14.5 cases per 100,000 patient-years. |
| Cabell et al16 | Retrospective analysis of IE incidence in the US Medicare database, reflecting more than 16,000 cases from 1986-1991. | 20.4 | This study reported a 13.7% increase in IE incidence in the Medicare population from 1986 to 1998. |
| Morellion and Que17 | Review of 26 publications between 1993 and 2003 encompassing 3784 cases of IE. | Median incidence of IE among all studies of 3.6. | In subjects older than 65 years, the median incidence was >15 cases per 100,000 person-years, nearly 3 times that of subjects less than 50 years of age. |
The incidence of IE among the elderly, often due to nosocomial infection, appears to be rising.19 A heightened index of suspicion is required to make the diagnosis in this population because its presentation may be atypical.20 There is a higher incidence of degenerative, calcific valve disease, which can decrease the specificity of echocardiographic imaging. Transesophageal echocardiography (TEE) imaging is often required for more accurate delineation of valve pathology.21 IE due to Enterococcus faecalis may be more common among the elderly.22 Although there are conflicting reports, advanced age appears to predict mortality in IE,19,23-25 particularly with Staphylococcus aureus infection.26
IE is a dreaded complication of injection (ie, intravenous or subcutaneous) drug use. The incidence of IE among injection drug users (IDUs) is approximately 2% to 5% per year, and IE is responsible for 5% to 20% of hospital admissions and 5% to 10% of overall mortality in this group.27 The incidence of IE and the causative agents in this population are likely related to contaminated injection technique (eg, sharing or licking of hypodermic needles), the injection of unsterile particulate material (eg, talcum), and the high prevalence of HIV infection. S. aureus is the most common etiologic agent and causes more than 60% of IE cases in IDUs.27 IE due to gram-negative bacilli (notably Pseudomonas aeruginosa) and fungi is also more common among IDUs. The distinctive feature of IE in IDU is that it frequently involves the right heart, with 60% to 70% of cases involving the tricuspid valve. The tricuspid valve may be particularly susceptible to bacterial infection due to chronic degenerative changes caused by the repetitive injection of irritants (eg, talcum) into peripheral veins. Septic pulmonary emboli are common. Despite the high frequency of S. aureus infection, isolated right heart involvement in IDUs partially explains the lower mortality in this population (4%) compared with other IE patient subsets. Nevertheless, left-sided infection does occur and can result in major complications, such as systemic emboli, paravalvular abscess, and severe valvular destruction. IDUs with uncomplicated right-sided IE may be eligible for short-course parenteral antibiotic therapy (2-4 weeks), a regimen that would not be considered in patients with left-sided IE.28 Because the administration of a 4-week parenteral antibiotic regimen in IDUs can be challenging, combination oral therapy with ciprofloxacin plus rifampin can be used safely and effectively in selected patients with uncomplicated right-sided staphylococcal IE.29 Given the relative efficacy of antibiotic cure for isolated right-sided IE in IDUs, surgical therapy is infrequently indicated and is reserved for cases of severe right heart failure due to tricuspid regurgitation, refractory bacteremia, and persistent tricuspid vegetations >20 mm with recurrent septic pulmonary emboli.30 IDU is not an absolute contraindication to surgery for patients with IE, and studies have shown that surgery can be performed in these patients safely and with acceptable outcomes.31
Although HIV infection and intravenous drug use commonly coexist, HIV infection appears to be an independent risk factor for the development of IE.32 Patients with low CD4 counts (<200 cells/mL) tend to have an increased risk of IE,33 as well as a higher associated mortality.27,34 IDUs who are HIV-positive have a higher incidence of left-sided valvular involvement and complications than HIV-negative IDUs.34 Although they rarely cause IE, Bartonella species can cause opportunistic infections, including IE, in patients with AIDS.35 There is a paucity of data on the characteristics of IE in the non–HIV-infected immunocompromised host. However, immunocompromised patients with gram-negative bacteremia or fungemia may be at risk for IE due to these organisms.
There are approximately 300,000 patients in the United States who are receiving hemodialysis (HD). Infectious complications of vascular access are a major cause of morbidity and mortality in HD patients. In this population there is an alarming rate of bacteremia, approaching nearly 1.0 episode per 100 patient-care months. Few other medical conditions, with the exception of chemotherapy-induced neutropenia and intravenous drug use, are associated with such high rates of bacteremia. As a result, IE occurs in approximately 2% to 6% of patients receiving HD. Staphylococcal species are the predominant organisms and gain access to the bloodstream via infected central venous catheters, arteriovenous (AV) grafts, or AV fistulas. Primary AV fistulas have the lowest rates of infections and are the access of choice whenever vascular anatomy allows. Indwelling central venous catheters have the highest rate of infections and are often associated with more serious metastatic complications.36 Access-related infections are the most common cause for the loss of dialysis vascular access and cause nearly 10% of deaths (second to only ischemic heart disease) in this patient population.37 Patients with end-stage renal disease have a high incidence of calcific degeneration of the aortic and mitral valves,38 which may promote bacterial colonization of the endocardium. Such degenerative valvular disease may render echocardiographic detection of vegetations extremely challenging.21 Vancomycin is often used as a first-line agent due to the high incidence of methicillin-resistant S. aureus (MRSA) in this population and the ease of administration of this drug with HD. Treatment of catheter-related bloodstream infections with antibiotics alone yields poor results, and removal of infected catheters or grafts is necessary in most circumstances.39 Effective prevention of bacteremia in the HD population hinges on early referral for primary AV fistula placement (preferably before dialysis is necessary) and strict adherence to sterile technique with access catheter manipulation.
The rates of pacemaker and implantable cardiac defibrillator (ICD) placement have dramatically increased over the past 10 years, given the growing number of evidence-based indications for their use. However, there is an increasing rate of device infection that appears to be disproportionate to the increased rate of device implantation.40 The overall incidence of cardiac device–related infective endocarditis (CDRIE) lies between that of native valve endocarditis and prosthetic valve endocarditis.41,42 Staphylococcal species are the causative organisms in more than 70% of cases.43,44 Infection with coagulase-negative staphylococci can be especially difficult to eradicate. The large majority of cardiac device infections are likely due to pocket site contamination at the time of device placement. Hematogenous seeding from a distant focus of infection, particularly one due to S. aureus, can cause late-onset infection. The clinical presentation may be misleading, initially with prominent respiratory or rheumatologic symptoms, as well as local signs of infection.44 Septic pulmonary embolism is a frequent complication and contributes to the high mortality associated with CDRIE.45 CDRIE is one of the most difficult forms of IE to diagnose; the Duke criteria in this setting lack adequate sensitivity. Modifications of the Duke criteria have been proposed to include signs of local (generator pocket, subclavian vein) infection and septic pulmonary embolism as major criteria.44,46 There are several important imaging considerations in patients with suspected intracardiac device infections. With transthoracic echocardiography (TTE), it can be difficult to differentiate infected vegetations from noninfected device-related thrombus. TEE is often required for adequate visualization, and it is essential that echocardiographic data be integrated with clinical and microbiologic data. The device and leads should be imaged throughout their entire course within the cardiac chambers and proximal veins, with special attention to their relationship to the tricuspid valve. Device leads that track through the superior vena cava should be visualized as close to the origin of the generator as possible.18 In addition to TEE, preliminary experience with intracardiac echocardiography for the detection of lead vegetations has recently been reported.47 Although no prospective studies have been conducted, management with parenteral antibiotics and complete device removal are the standards of care.43 Cardiac device extraction can be performed percutaneously without the need for surgical intervention in the majority of patients, although percutaneous approaches may be more difficult when the device has been in place for several years. Pulmonary embolism of vegetative material during extraction occurs frequently, particularly when vegetations are large.46,48 However, these episodes are frequently asymptomatic, and percutaneous extraction remains the procedure of first choice, even in cases with large vegetations,45,46,48 because the overall risks are even higher with surgical extraction.30,44 Increased experience with evolving percutaneous lead-extraction techniques may aid in the management of infected devices.49 When there is documented valvular IE in a patient with an implanted device, there is high likelihood of concomitant device infection, which usually necessitates extraction at the time of valve surgery. The American Heart Association has recently published a scientific statement that provides recommendations for the prevention, diagnosis, and management of cardiovascular implantable electronic device (CIED) infections.50 Decisions regarding optimal timing and site of device reimplantation are complex and must be adapted to the individual patient.30 A retrospective analysis of patients undergoing pacemaker implantation suggests that antibiotic prophylaxis before device implantation can prevent infectious complications.51 The current scientific statement recommends that prophylaxis with an antistaphylococcal agent should be administered at the time of CIED placement.50 Antimicrobial prophylaxis for the prevention of CIED infections is not recommended for dental or other invasive procedures not directly related to device manipulation.50
Pathogenesis
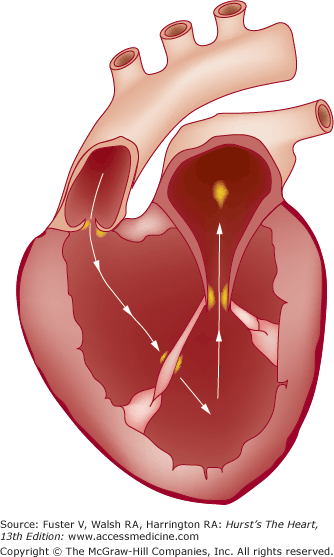
The hallmark of IE is persistent endocardial or endovascular infection causing continuous bacteremia. Importantly, IE is a relatively uncommon consequence of transient bacteremia, and not all organisms can effectively colonize or invade the endovascular space. It is apparent that a complex series of host-pathogen interactions conspire in the development of IE lesions, including the integrity of the vascular endothelium, the host immune system, hemostatic mechanisms, cardiac anatomical characteristics, microbial properties, and the peripheral events that cause the bacteremia.18,52 There are substantial experimental data to suggest that host endothelial damage is the key predisposing insult, with subsequent platelet and fibrin deposition and creation of a receptive milieu for bacterial colonization during episodes of transient bacteremia. That endothelial damage is the inciting event is further supported by the observation that vegetations are most likely to form in areas where blood-flow injury is likely to occur—on the ventricular side of semilunar valves and the atrial side of AV valves.53 Jet lesions from regurgitant valves or intracardiac shunts may also damage endothelium, and vegetations may form on such sites of injury, including the mitral chordae with aortic regurgitation, the mural left atrial endocardium with mitral regurgitation, or the septal leaflet of the tricuspid valve with ventricular septal defect.18,53Figure 86–1 illustrates the classic locations of endocardial flow injury that are associated with vegetation formation in IE.53
Figure 86–1.
Sites of typical endocardial flow-related injury and associated vegetation formation in infective endocarditis (IE). Flow injury from aortic regurgitation is typically associated with vegetations on the ventricular side of the aortic valve or on the anterior mitral leaflet and its subvalvular apparatus. Flow injury from mitral regurgitation is associated with vegetation formation on the left atrial surface of the mitral leaflets or on the mural surface of the left atrial endocardium (MacCallum patch). Flow injury from a ventricular septal defect (not shown) with left-to-right shunting is associated with vegetation formation on the septal leaflet of the tricuspid valve. Adapted from Rodbard.53
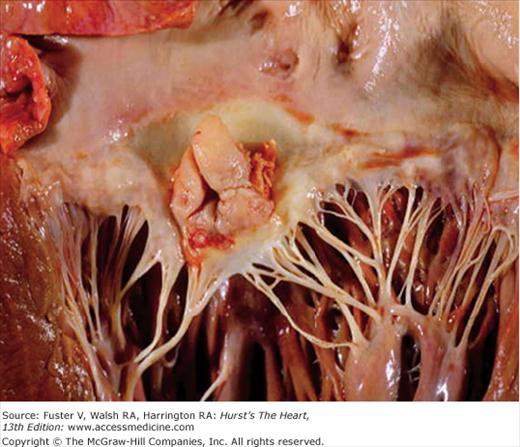
Once endothelial injury has occurred, bacteria must gain access to the intravascular space to seed the lesion. Common sources of bacteremia include skin, surgical wounds, the periodontal space, indwelling intravascular catheters, and the urinary and gastrointestinal tracts. Bacteria that have entered the bloodstream must be able to adhere to the damaged endothelium, exposed extracellular matrix, or areas of fibrin deposition.54 This adherence is mediated by microbial surface components recognizing adhesive matrix molecules, which recognize a variety of host proteins such as fibronectin, collagen, and integrins. Certain organisms appear to preferentially express these molecules, enabling them to adhere more effectively and colonize the injured endocardium.52,55 The fact that S. aureus can induce endothelial tissue factor expression may partially explain why this organism can adhere to relatively normal heart valves.56 Particulate material that may be injected may also promote S. aureus adherence by stimulating matrix protein expression on valvular endothelium.54 Another factor in the pathogenesis of device-related intravascular infections is biofilm formation, which impedes microbial clearance by host mechanisms and thus often mandates device removal for eradication of the infection. S. epidermidis and S. aureus have been studied most extensively in this regard, with characterization of a responsible intercellular adhesin and its gene cluster.57 Once adherent, certain strains of bacteria are able to evade the host immune system, destroy host tissue, or occasionally enter the cytosolic compartment of endothelial cells. Repetitive cycles of bacterial proliferation, fibrin-platelet deposition, and host tissue destruction create an infected vegetation, within which bacteria can reach extremely high concentrations (109-1011 organisms per gram of tissue; Fig. 86–2). These dense vegetations are a source of continuous bacterial seeding of the bloodstream. Figure 86–3 shows a gross pathologic specimen of the heart with a large, complex vegetation attached to the left atrial surface of the anterior mitral leaflet. After successful antimicrobial therapy, vegetations will resolve completely or contract in size and persist indefinitely as a sterile and frequently calcified mass adherent to valve tissue.
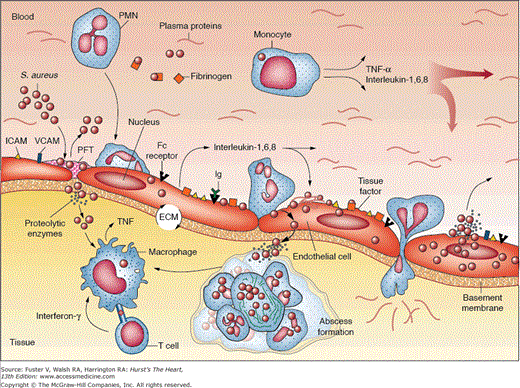
Figure 86–2.
Pathogenesis of staphylococcal infective endocarditis. ECM, extracellular matrix; ICAM, intercellular adhesion molecule; PFT, platelet-fibrin thrombus; PMN, polymorphonuclear leukocyte; TNF-α, tumor necrosis factor α; VCAM, vascular cell adhesion molecule. Adapted with permission from Lowy F. Staphylococcus aureus infections. N Engl J Med. 1998;339:520-532.
Microbiology of IE
A wide range of microorganisms can cause IE, but only a few species account for the vast majority of cases. Streptococci and staphylococci are the cause of greater than 80% of IE cases in which a responsible organism is identified. Streptococcal species were historically the most common group of pathogens, but more recent data identify S. aureus as the most frequently isolated microbial agent world wide.26 Moreover, the rate of antibiotic resistance among causative organisms is increasing.26,58
Viridans group streptococci, or α-hemolytic streptococci, are a frequent cause of community-acquired native valve endocarditis (NVE). Viridans streptococci are responsible for 30% to 65% of cases of NVE in older children and adults. They are normal residents of the oropharynx and easily gain access to the circulation after dental or gingival trauma. The viridans streptococci comprise several species, of which S. sanguis, S. bovis, S. mutans, and S. mitior are most commonly isolated in cases of IE. The viridans streptococci are usually highly sensitive to penicillin, as defined by a minimum inhibitory concentration of <0.1 μg/mL, and thus can often be eradicated with penicillin monotherapy.59
S. bovis, a normal inhabitant of the human gastrointestinal tract, is noteworthy because IE caused by this organism is strongly suggestive of gastrointestinal (GI) malignancy,60 polyp formation, or diverticular disease. Colonoscopy should be performed when this organism is detected in the blood. When meticulously investigated, GI pathology is discovered in as many as 60% of patients with S. bovis IE.60
Nutritionally deficient streptococci, now known as Abiotrophia spp. and Granulicatella spp., require specialized isolation and culture techniques for growth (supplemental thiol compounds or active forms of vitamin B6) and now account for approximately 5% to 7% of streptococcal IE cases.59,61 IE due to these organisms is virtually always indolent in onset and associated with preexisting heart disease. Therapy remains difficult and prognosis is poor because these organisms are generally less sensitive to penicillin than the viridans group streptococci. Treatment often requires synergistic therapy with an aminoglycoside.
The Enterococcus spp., formerly classified as group D streptococci, are now defined as a distinct genus. They are responsible for 5% to 18% of cases of native valve IE, the vast majority of which are due to E. faecalis (80%) or E. faecium (10%). These organisms are normal inhabitants of the GI and genitourinary tracts and may enter the bloodstream after manipulation of the colon, urethra, or bladder (eg, Foley catheterization, colonoscopy). The incidence of enterococcal endocarditis appears to be rising, likely due to the increased genitourinary and GI instrumentation in older adults and the increased use of indwelling central venous catheters and prosthetic implants. These organisms can infect both normal and diseased heart tissue, as well as prosthetic materials. Indeed, among the most relevant risk factors for development of enterococcal IE is the presence of an implanted device. The pathogenesis of such infections is poorly understood, but several virulence factors have been proposed. The ability to form biofilm has recently been shown to be one of the most prominent features of this microorganism, allowing colonization of inert and biological surfaces and preventing antibiotic penetrance. The disease typically runs an indolent course and cure is challenging because the organism has limited susceptibility to many antibiotics, including β-lactam drugs. There is an alarming incidence of nosocomial bacteremia with these organisms and a growing problem with drug resistance (to both vancomycin and aminoglycosides). Although synergistic antibiotic therapy, as predicated by susceptibility testing, should be considered,58 there is some debate regarding its efficacy.62
Group A Streptococcus rarely causes IE. S. pyogenes, the causative organism in childhood pharyngitis, scarlet fever, impetigo, cellulitis, erysipelas, fasciitis, and myositis, is not associated with IE in adults. Before 1945, S. pneumoniae caused approximately 10% of IE cases; however, the current incidence of pneumococcal IE is very low. S. pneumoniae bacteremia often begins with respiratory infection, and nearly half of patients with pneumococcal IE suffer from chronic alcoholism.63S. pneumoniae can infect normal valve tissue and usually results in an acute, fulminant illness often associated with severe valve damage, perivalvular extension, embolic complications, pericarditis, meningitis, and high mortality (25%-50 %).63
Group B streptococci (eg, S. agalactiae) are chiefly responsible for infections in the neonate and parturient, although these organisms can be isolated from diabetic foot ulcers. Risk factors for group B streptococcal bacteremia in adults include obstetric complications,64 diabetes, carcinoma, liver failure, alcoholism, and IDU.65 These organisms are generally less sensitive to penicillin than group A isolates and require higher doses for treatment.
S. aureus causes 80% to 90% of staphylococcal IE and is the most common cause of acute IE. Recent prospective data from the International Collaboration on Endocarditis (ICE) suggest that S. aureus has become the leading cause of IE worldwide and more often presents as an acute disease.2,26 The mucous membranes of the anterior nasopharynx are the most common sites of colonization, and approximately 30% of normal persons carry S. aureus. Carrier rates are higher among persons with more frequent exposures to the organism and those who are at risk for breakdown of the normal mucocutaneous barrier. Rates of S. aureus infection, particularly bacteremia associated with health care contact, have increased in hospitalized patients and among those receiving outpatient medical therapy.66,67 Although only a fraction of patients with S. aureus bacteremia will develop NVE,68 populations at increased risk include patients on dialysis,37 patients with type I diabetes, burn victims, persons with HIV,32 IDUs,27 patients with certain chronic dermatologic conditions, and patients with recent surgical incisions (including median sternotomy for valve replacement). Despite the frequency of nosocomial S. aureus acquisition, community-acquired infection appears to be an independent risk factor for the development of IE and metastatic disease.26,69S. aureus is respected as a highly virulent organism and has the capacity to infect and destroy normal endocardial surfaces (see Fig. 86–2). A number of virulence factors help this organism enter the bloodstream, adhere to endothelial and prosthetic surfaces, and evade host defense.55S. aureus IE is frequently fulminant when it involves left-sided cardiac valves and often results in major complications such as valve destruction, heart failure, perivalvular extension with conduction disturbances, embolization, and metastatic infection.70 Not surprisingly, S. aureus as a causative organism is an independent predictor of poor prognosis in IE71 and is associated with a 25% to 30% mortality.1,26 As many as 50% of patients with left-sided native valve IE (NVE) due to S. aureus will require surgery. Right-sided (tricuspid valve) IE with S. aureus, by contrast, is most frequently associated with IDU and is associated with a high incidence of septic pulmonary embolization, but only a 2% to 4% case fatality rate. The detection of S. aureus bacteremia should prompt echocardiography to look for evidence of IE, especially if bacteremia is persistent. Although most patients undergo TTE first, an initial TEE should be considered for patients with catheter-associated S. aureus bacteremia as a cost-effective means of determining the duration of antibiotic therapy (ie, 2 weeks vs 4 weeks).72 A semi-synthetic, β-lactamase-resistant penicillin (eg, nafcillin) is preferred for initial treatment of susceptible S. aureus in nonpenicillin allergic individuals, often with a short course of an aminoglycoside. Antibiotic resistance can be a particular challenge with S. aureus because more than 90% of clinical isolates produce β-lactamase and are thus penicillin-resistant. Semisynthetic penicillin analogs that are unaffected by β-lactamase, such as methicillin, oxacillin, and nafcillin, are first-line agents. Increasing rates of MRSA in both hospital and community settings,26 and the recovery of clinical S. aureus isolates resistant to vancomycin,73 have complicated the treatment of S. aureus IE. Increasing hospital use of vancomycin for the treatment of MRSA is likely one additional reason for the growing incidence of vancomycin resistance among enterococci. Vancomycin, although the best available antibiotic for treatment of MRSA, is only weakly bactericidal and predominantly bacteriostatic. Recently, S. aureus isolates with varying levels of vancomycin resistance have been cultured from infected patients and have been associated with IE treatment falures.74 The new lipopeptide antistaphylococcal agent daptomycin has been approved for treatment of S. aureus bacteremia and right-sided IE75 and may have promise for the treatment of left-sided IE due to resistant organisms. Cases of resistant S. aureus IE should always be managed in close collaboration with an infectious diseases specialist.
The coagulase negative staphylococci (CoNS) are constituents of normal human skin flora and are much less likely to infect normal endocardial surfaces. S. epidermidis is an important causative agent in prosthetic valve and device-related endocarditis. Native valve endocarditis caused by CoNS occurs mainly in patients with preexisting valvular heart disease,76 though exceptions to this generalization do occur (see Fig. 86–3). NVE caused by CoNS has historically been regarded as an indolent disease; however, recent data from the ICE have demonstrated equal rates of heart failure and death for CoNS and S. aureus NVE.76S. epidermidis infections of intracardiac devices are extremely difficult to eradicate, and the addition of adjunctive course of rifampin therapy is often recommended. Rare cases of IE due to other CoNS (e.g. S. saprophyticus, S. capitis) have been reported 77,78, including a growing number of reports of IE caused by community acquired S. lugdunensis.79S. lugdunensis may cause a more virulent form of IE with high morbidity despite uniform in vitro susceptibility to most antibiotics.
IE due to gram-negative bacilli is uncommon and tends to occur in IDUs, immunocompromised patients, patients with advanced liver disease, and prosthetic heart valve recipients. The fastidious gram-negative rods of the HACEK group (Haemophilus spp., Actinobacillus, Cardiobacterium hominis, Eikenella corrodens, and Kingella spp.) reside normally in the oropharynx and are responsible for a very small (approximately 1%) proportion of cases of NVE, usually involving abnormal valve tissue. Because of their growth requirements (CO2), they may take 3 to 4 weeks to grow in culture and have gained notoriety for their implicated role in certain cases of culture-negative IE. The Haemophilus species (H. parainfluenzae, H. aphrophilus, H. paraphrophilus) are the most common etiologic agents from this group. They typically form large and friable vegetations that have a tendency to embolize. There are numerous case reports of IE caused by other members of the HACEK group.80 The Enterobacteriaceae (E. coli, Klebsiella, Enterobacter, Serratia, etc) are rare causes of IE. Salmonella species have a particular predilection for atherosclerotic plaque and may infect arterial aneurysms.81 The vast majority of patients with P. aeruginosa endocarditis are IDUs.82 The source of Pseudomonas appears to be from standing water that contaminates needles and other drug paraphernalia. Left-sided endocarditis caused by P. aeruginosa is difficult to eradicate with antibiotics alone, has a high rate of complications, and often requires early surgery.82
The rickettsial organism, Coxiella burnetii, is the causative agent of Q fever and is a relevant cause of IE in areas where cattle, sheep, and goat farming are common. Cases of IE caused by C. burnetii are well documented in the developed world.83 The aortic valve is affected in more than 80% of cases, and the infection is difficult to eradicate with antibiotics. As the organism is extremely difficult to culture, the diagnosis is best made serologically using antibody titers.80Bartonella species (quintana, henselae, elizabethae), the etiologic agents in cat scratch disease, have been recently described as an important cause of IE among both homeless men and HIV-infected patients. The diagnosis can be suspected serologically and confirmed with special culture techniques or by polymerase chain reaction.84Brucellae have been implicated in approximately 4% of cases of IE in Spain. These organisms are usually ingested in unpasteurized milk or cheese and are occupational hazards for veterinarians, shepherds, and livestock handlers. IE is the most common cause of death in patients with brucellosis, and surgery is usually required for cure.85
Fungal endocarditis is a relatively new syndrome associated with exceedingly high mortality (survival rates of <20%). Patients who develop fungal IE have multiple predisposing conditions that include an immunocompromised state, the use of endovascular devices, and previous reconstructive cardiac surgery. Candida and Aspergillus species are the most common causes of fungal IE and are associated with large, bulky vegetations that can obstruct valve orifices and can also embolize to large vessels (eg, the femoral artery). Blood cultures are usually positive in cases of Candida IE, whereas they are rarely positive with Aspergillus. Fungal endocarditis is an indication for surgical replacement of an infected valve. Cure usually requires combination fungicidal (amphotericin) and surgical treatment, followed by long-term suppressive therapy with an oral antifungal agent.58
Blood cultures are negative in up to 20% of patients with IE diagnosed by strict criteria.86 Failure to isolate a microorganism may be the result of inadequate culture technique, a highly fastidious organism or a nonbacterial pathogen as the causative agent, or previous administration of antimicrobial therapy before blood culture acquisition. The latter is an extremely important consideration because the administration of antibiotics before drawing blood cultures can reduce the recovery rate of bacterial pathogens by nearly one-third.87,88 There are numerous noninfectious causes of endocarditis that may behave like culture-negative IE, including those that are related to neoplasia (nonbacterial thrombotic endocarditis), autoimmune diseases (antiphospholipid antibody syndrome, systemic lupus erythematosus [SLE]), or the postcardiac surgical state (thrombi, sutures).88 Empiric therapy for culture-negative IE remains extremely challenging because the patient may be exposed to potentially toxic antimicrobial therapy while awaiting what is often delayed identification of a causative organism, if one is identified at all. Consultation with an infectious disease specialist to define the most appropriate therapy is recommended.58
Prosthetic valve endocarditis (PVE) represents approximately 10% to 30% of all IE cases.9,15,52 Although many of the general principles applicable to native valve IE are relevant, there are important considerations specific to PVE. After valve replacement, the incidence of PVE is approximately 1% to 3% at 1 year and 3% to 6% at 5 years. Although the current evidence is not definitive, PVE can be broadly divided into two groups based on the time of onset of the infection after valve surgery—early PVE and late PVE. Early PVE is defined as endocarditis that develops within the first 2 months to 1 year after valve surgery. During this period, the vast majority of causative organisms are nosocomially acquired with a predominance of Staphylococci, notably coagulase-negative species (S. epidermidis). Gram negative bacilli, diphtheroids, and fungal species, although very uncommon causes of IE overall, have a predilection to cause PVE during this early period. Late PVE is defined as that occurring beyond 1 year postoperatively. In contrast to early PVE, the spectrum of causative organisms in late PVE resembles that of NVE. In late PVE, there is an emergence of cases due to streptococci and enterococci, a significant decrease in the rate of coagulase-negative staphylococci, and a continued rate of infection with S. aureus. Between 2 and 12 months after valve replacement surgery, there is a gradual transition between the early and late microbiologic causes of PVE. TEE imaging is recommended in cases of suspected PVE (see below). In some cases, both TTE and TEE are performed to provide optimal characterization of the infection, prosthetic valve function, and cardiac performance. Empiric therapy for suspected PVE is similar to that for NVE; however, staphylococcal coverage should always be provided until definitive culture data are available, with particular attention to the likelihood of antibiotic resistance (MRSA). Complication rates with PVE are high and surgery is often required even in the absence of documented perivalvular extension. For example, S. aureus PVE is rarely eradicated with antibiotics alone, and retrospective analyses suggest that combined medical and surgical therapy is more effective than medical therapy alone.89 With proper timing of antibiotic therapy and surgery, the imputed risk of early reinfection of a newly implanted valve is only 2% to 3%.90
Approach to the Patient with Suspected IE
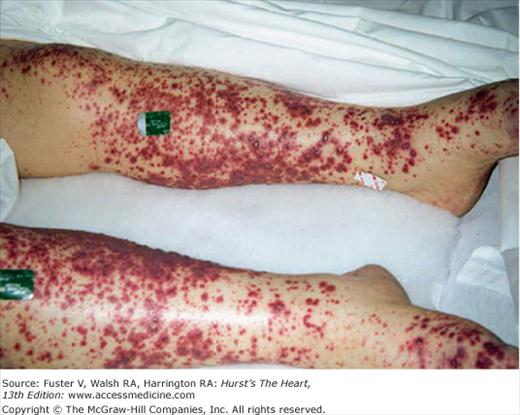
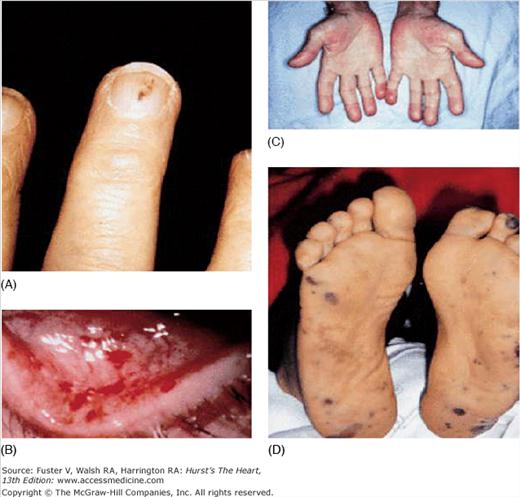
The history should focus on predisposing factors such as IDU, a prior history of IE, recent exposures, the presence of an intracardiac device or indwelling central venous catheter, congenital or acquired valvular heart disease, and other congenital or structural heart disease. The patient may report fever, fatigue, anorexia, weight loss, night sweats, joint pain, or back pain. Often, the nature of the presenting symptoms reflects the severity and type of infection. Patients with S. aureus infection, for example, are likely to have a fulminant course with high fever and systemic sepsis, whereas some infections with streptococcal organisms may have a more protracted course. Features on clinical examination that raise suspicion for IE include fever, a new heart murmur indicative of valvular insufficiency, signs of heart failure, and vascular phenomena. Examples of classic IE-related findings include major arterial emboli with pulse deficits, septic pulmonary emboli (with right-sided IE), mycotic brain aneurysms with intracranial hemorrhage, mucosal or conjunctival petechiae, splinter hemorrhages of the nail beds, palpable purpuric skin rashes (Fig. 86–4), Janeway lesions (small, flat, irregular erythematous spots on the palms and soles), Osler nodes (tender, erythematous nodules occurring in the of the fingers), Roth spots (cytoid bodies and associated hemorrhage caused by micro-infarction of retinal vessels), and urinary red cell casts suggestive of glomerulonephritis (Fig. 86–5).
Figure 86–5.
Selected peripheral manifestations of infective endocarditis. A. Splinter hemorrhages are linear hemorrhages under the nails that do not reach the nail margin. They are often red for the first two days and brownish thereafter. B. Conjunctival hemorrhages. C. Osler nodes are tender, erythematous nodules often occurring in the pulp of the fingers. D. Janeway lesions are small, flat, irregular spots found on the palms and soles. They are typically erythematous and nontender. Adapted with permission from Mylonakis and Calderwood.10
Although the history and physical examination are useful, the diagnosis of IE rests on the ability to demonstrate endocardial involvement and persistent bacteremia. The proper acquisition of blood cultures before initiation of antimicrobial therapy is essential. In hospitalized patients, the presence of bacteremia with a typical causative organism (eg, S. aureus) often provides initial suspicion for IE and prompts further diagnostic evaluation. Echocardiography should be used to assess for the presence of endocardial involvement (vegetations, abscess formation, and new valvular regurgitation). These clinical, microbiologic, and echocardiographic features are the foundation for the modified Duke criteria, a set of integrated findings that has become the standard for diagnosis of IE (Tables 86–2 and 86–3).91 These criteria are discussed in detail later.
| Major criteria |
| Blood culture positive for IE |
| Typical microorganisms consistent with IE from 2 separate blood cultures: |
| Viridans streptococci, Streptococcus bovis, HACEK group, Staphylococcus aureus; or |
| Community-acquired enterococci in the absence of a primary focus; or |
| Microorganisms consistent with IE from persistently positive blood cultures, defined as follows: |
| At least 2 positive cultures of blood samples drawn more than 12 h apart; or |
| All of 3 or a majority of greater than 4 separate cultures of blood (with first and last sample drawn at least 1 h apart) |
| Single positive blood culture for Coxiella brunetti or anti-phase 1 IgG antibody titer greater than 1:800 |
| Evidence of endocardial involvement |
| Echocardiogram positive for IE (TEE recommended in patients with prosthetic valves, rated at least “possible IE” by clinical criteria, or complicated IE [paravalvular abscess]; TTE as first test in other patients), defined as follows: |
| Oscillating intracardiac mass on valve or supporting structures, in the path of regurgitant jets, or on implanted material in the absence of an alternative anatomic explanation; or |
| Abscess; or |
| New partial dehiscence of prosthetic valve. |
| New valvular regurgitation (worsening or changing of preexisting murmur not sufficient) |
| Minor criteria |
| Predisposition, predisposing heart condition, or injection drug use |
| Fever, temperature greater than 100.4°F (38°C) |
| Vascular phenomena, major arterial emboli, septic pulmonary infarcts, mycotic aneurysm, intracranial hemorrhage, conjunctival hemorrhages, and Janeway lesions |
| Immunologic phenomena; glomerulonephritis, Osler nodes, Roth spots, and rheumatoid factor |
| Microbiologic evidence: positive blood culture but does not meet a major criterion,a or serologic evidence of active infection with organism consistent with IE |
| Echocardiographic minor criteria eliminated |
| Definite infective endocarditis |
| Pathologic criteria |
| (1) Microorganisms demonstrated by culture or histologic examination of a vegetation, a vegetation that has embolized, or an intracardiac abscess specimen; or |
| (2) Pathologic lesions, vegetation, or intracardiac abscess confirmed by histologic examination showing active endocarditis |
| Clinical criteria |
| (1) 2 major criteria; or |
| (2) 1 major criterion and 3 minor criteria; or |
| (3) 5 minor criteria |
| Possible infective endocarditis |
| (1) 1 major criterion and 1 minor criterion; or |
| (2) 3 minor criteria |
| Rejected |
| (1) Firm alternate diagnosis explaining evidence of infective endocarditis; or |
| (2) Resolution of infective endocarditis syndrome with antibiotic therapy for less than 4 days; or |
| (3) No pathologic evidence of infective endocarditis at surgery or autopsy, with antibiotic therapy for less than 4 days; or |
| (4) Does not meet criteria for possible infective endocarditis, as noted above |
Once the diagnosis has been made and appropriate therapy initiated, the patient should be monitored closely for complications, especially during the first week of therapy. All patients should have repeat blood cultures after institution of antibiotics to ensure sterility. Persistent fever beyond 1 week of appropriate therapy should raise suspicion for intracardiac extension or satellite abscess formation. In the absence of complications, the first several days of intravenous antibiotics are administered in the hospital and the remaining course provided via a central venous catheter (eg, peripherally inserted central catheter [PICC]) as an outpatient with careful follow-up. Patients should be maintained on telemetry while in hospital; the need for surveillance ECGs during outpatient therapy is dictated by the location of the infection and the predicted likelihood of conduction disturbances. Patients should be monitored for antimicrobial toxicity, particularly with aminoglycoside use. Routine surveillance echocardiography during therapy is not necessary unless complications develop or cardiac surgery is considered (see later, Echocardiography in Infective Endocarditis). At the completion of therapy, TTE may be performed to establish a new “post-IE baseline.”58 After successful therapy, patients with IE should be followed longitudinally for progressive valvular and ventricular dysfunction. Patients with successfully treated IE are at high risk for the development of future episodes of IE and should receive antibiotic prophylaxis before specific dental procedures, as recommended by current guidelines (see later under Antibiotic Prophylaxis for the Prevention of IE).92,93
Diagnosis of Infective Endocarditis
IE is defined as an infection on any structure within the heart, including normal or damaged endothelial surfaces, prosthetic heart valves, and implanted devices (eg, pacemakers, ICDs, ventricular assist devices, and surgical shunts).18 The diagnosis of IE relies chiefly on the following factors: (1) an initial clinical suspicion, especially in a patient with identifiable risk factors; (2) microbiologic data (blood cultures demonstrating continuous bacteremia or cultures of vegetative emboli removed surgically); and (3) the results of echocardiographic imaging. Diagnosis is straightforward in only a minority of patients who present with a defined predisposing condition and the classic manifestations of fever, evidence of active valvulitis, peripheral emboli, immunologic or vascular phenomena, and bacteremia. In the majority of patients, IE has an extremely variable clinical presentation.2,59
Pelletier and Petersdorf94 published the first formal case definition based on their 30-year experience caring for patients with IE in Seattle, WA. Although this first case definition was specific for the diagnosis of IE, it lacked sufficient sensitivity. Subsequently, von Reyn and colleagues95 developed the first stratified diagnostic schema in which cases were placed into four categories (rejected, possible, probable, and definite). This approach eventually proved inadequate due to over-classification of cases as “possible” and “probable,” over-reliance on pathologic specimens, and the lack of incorporation of echocardiographic data. In 1994, Durack and colleagues published the Duke criteria96 for the diagnosis of IE that incorporated additional clinical factors and echocardiographic data. These criteria were revised in 2000 to increase the sensitivity for detection of cases related to S. aureus bacteremia, as well as to account for culture-negative IE.91 The modified Duke criteria (see Tables 86–2 and 86–3) have become the current standard for diagnosis and clinical research and have been validated in numerous subsequent studies.97-99 A diagnosis of “definite IE” is established clinically by evidence of two major criteria, one major plus three minor criteria, or five minor criteria. Patients identified with “possible IE” (one major plus one minor criterion or three minor criteria) should be treated for IE until the diagnosis is satisfactorily excluded. Application of these criteria in clinical practice will capture the vast majority of cases of IE and render the possibility of missing a potential case quite remote. The criteria are both clinically and biologically sound, relying on microbiologic data and evidence of endocardial involvement, with attention to predisposing factors and early complications of a vascular or immunologic nature. Definitions of specific criteria and terms used in the modified Duke criteria are detailed in Table 86–2.93
The importance of obtaining blood cultures by appropriate methods, before the institution of antibiotics, cannot be overemphasized. Three separate sets of blood cultures obtained from different venipuncture sites over 24 hours are recommended. For the most commonly encountered bacterial pathogens (staphylococci, streptococci, enterococci), the first two sets of blood cultures will be positive in the vast majority of cases.100 Culture-negative IE is most commonly associated with antecedent antibiotic use, but may be due to fastidious (eg, HACEK) or intracellular organisms that are not readily detected using standard culture techniques,80 especially if the laboratory has not been alerted to the possibility of IE and the need to perform additional testing. Identification of the offending pathogen may require special culture media and prolonged incubation times (>2 weeks).17 Additional indirect measures of active infection with difficult-to-culture organisms (eg, Bartonella, Brucella, Legionella) include positive results of serology, agglutination, immunofluorescence, and polymerase chain reaction amplification assays.59,80 It should be noted that many of these indirect diagnostic tests may be nonspecific, and careful interpretation is needed to avoid erroneous or false-positive results.
All patients with suspected IE should undergo prompt echocardiographic assessment. There are several TTE findings suggestive of endocarditis, including vegetations, evidence of peri-annular tissue destruction (ie, abscess formation), aneurysms or fistula formation, leaflet perforation, or prosthetic valve dehiscence.18 Specific definitions of these findings are outlined in Table 86–4.101 The echocardiographic definition of a vegetation is an irregular-shaped, discrete echogenic mass that must be adherent to, yet distinct from, the endothelial surface. The presence of a vegetation is strongly supported if the suspected mass oscillates at a high frequency, independent of other intracardiac structures.
| Finding | Description |
|---|---|
| Vegetation |