The relation between left atrial (LA) mechanics and left ventricular (LV) diastolic function and adverse cardiovascular events are not well established in chronic systolic heart failure (HF). In 108 patients, we performed comprehensive echocardiography with an assessment of LA global longitudinal strain (LAε) by Velocity Vector Imaging. We also performed complete diastolic examinations including mitral inflow, pulmonary vein flow, and tissue Doppler. Death, cardiac transplantation, and HF hospitalization were tracked for 5 years. In our study cohort (age 57 ± 15 years, LV ejection fraction 25 ± 6%), mean global LA negative (ε negative ), positive (ε positive ), and total ε (ε total ) were −6.8 ± 4.4%, 7.7 ± 5.7%, and 14.5 ± 8.2%, respectively. All LAε indexes correlated with individual indexes of LV diastolic dysfunction, including mitral flow early (E) to late diastolic velocity ratio (p <0.05 for all), mitral deceleration time (p <0.01 for all), E to early diastolic velocity of the septal mitral annulus (e′) ratio (p <0.05 for all), pulmonary vein flow systolic to diastolic velocity ratio (p <0.001 for all), and maximal LA volume index (p <0.01 for all). All LAε indexes increased across diastolic stage (p <0.001 for all). In multivariate logistic regression analysis, LAε negative and LAε total were associated with the presence of LV diastolic dysfunction grade III even after adjustment for E/e′ septal and LA volume index. In Cox proportional hazards analysis, lower magnitude LAε negative predicted long-term adverse clinical events. In conclusion, more impaired LA mechanics are associated with more severe diastolic dysfunction and predict long-term adverse events in patients with chronic systolic HF.
There has been increasing interest in the noninvasive evaluation of the left atrial (LA) size and phasic function, which can significantly affect overall cardiac performance and cardiovascular outcomes. Recently developed 2-dimensional strain based on speckle tracking is an innovative method providing insight into myocardial mechanics. The evaluation of LA function using this technique has been widely accepted and as assessed with cardiac magnetic resonance imaging, LA strain (ε) has been related to LA structural remodeling and fibrosis of the atrial wall. Growing evidence suggests that ventricular myocardial mechanics provide incremental prognostic information over standard parameters in population at risk for or with heart failure (HF). However, the long-term prognostic value of deformation-based parameters of LA function and its relation with standard clinical markers, especially with left ventricular (LV) diastolic parameters, in patients with chronic systolic HF have not been well established. We sought to examine the ability of LA global longitudinal strain (LAε) to assess the impact of LV diastolic function on LA mechanics and disease severity in patients with chronic systolic HF.
Methods
The Assessment of Doppler Echocardiography Prognosis and Therapy (ADEPT) study was a prospective, single-center, cohort study of patients with chronic systolic HF (New York Heart Association functional classes II to IV) seen at the outpatient cardiology clinics at Cleveland Clinic from May 1, 2001 to June 30, 2003. Eligible patients were 18 to 75 years of age with an LV ejection fraction ≤35%. Patients who had mitral stenosis or mitral valve surgery, severe mitral regurgitation (>3), severe aortic stenosis (peak velocity >4 m/s) or regurgitation, significant hepatic or renal dysfunction, or atrial fibrillation during echocardiographic examination were excluded. This study was approved by the Cleveland Clinic Institutional Review Board. Informed consent was obtained from all subjects. Patients underwent echocardiographic evaluation of myocardial systolic and diastolic performance. We assessed the relation between LV diastolic dysfunction and LA mechanics. Adverse events (all-cause mortality, cardiac transplantation, or HF hospitalization) were prospectively tracked for 5 years by scheduled telephone follow-up and validated by chart review as previously described.
Comprehensive transthoracic echocardiography was performed by highly experienced research sonographers using commercially available HDI 5000 (Phillips Medical Systems, Bothell, Washington) and Acuson Sequoia (Siemens Medical Solutions USA Inc., Malvern, Pennsylvania) machines. Doppler imaging and 2-dimensional imaging were performed in standard parasternal and apical views. LV systolic and diastolic indexes were acquired as previously outlined in the ADEPT trial. All images were stored digitally and were measured with offline software (Syngo Dynamics 9.0 software; Siemens Medical Solutions). Overall diastolic stage, determined from transmitral and pulmonary vein flow patterns, was defined as abnormal relaxation (stage I, transmitral flow early (E) to late (A) diastolic velocity ratio <1, and atrial reversal <35 cm/s), pseudonormal (stage II, E/A ratio 1 to 2, transmitral flow deceleration time 150 to 200 ms, pulmonary vein flow systolic to diastolic (S/D) ratio ≤1, and atrial reversal >35 cm/s), or restrictive (stage III, E/A ratio >2, deceleration time <150 ms, S/D ratio <1, and atrial reversal >35 cm/s). The LV ejection fraction and maximal LA volume index (LAVi) were measured using the Simpson biplane method. Measurements were averaged over 3 cycles, and 2 experienced individual research personnel (AGB and MGM) who were blinded from the clinical data made all measurements at the time of original ADEPT trial.
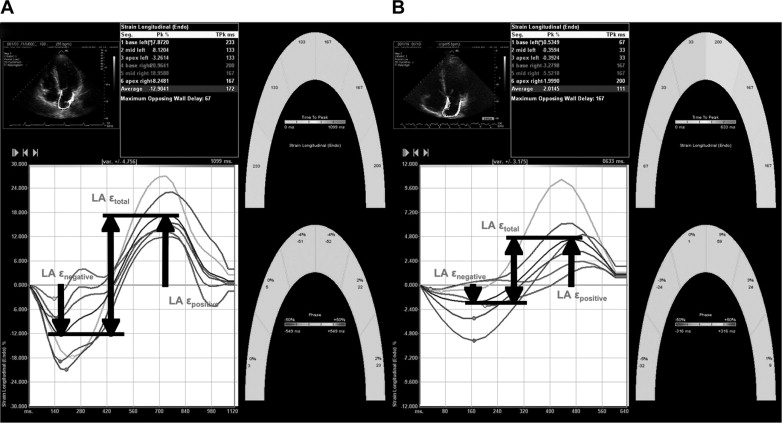
LAε measurements were performed offline using a dedicated software (Velocity Vector Imaging, Siemens Medical Solutions) in 2012. We used the onset of the P wave as the reference point for the calculation of LAε, as previously proposed. One cardiac cycle was selected for apical 4- and 2-chamber views, the endocardial border was traced manually in the end-systolic frame, and the software subsequently and automatically traced the borders in the other frames. In segments with poor tracking (assessed subjectively), endocardial borders were readjusted until optimal tracking was achieved. If this was unattainable, that segment was excluded. Graphical displays of deformation parameters for each segment were then generated automatically and were used for measurement of LAε values ( Figure 1 ). We obtained LAε only in the case of adequate tracking quality ≥5 of the 6 segments/view. Any view in which ≥2 segments could not be tracked was not included in the analysis, and the remaining apical views were averaged to calculate LAε. We identified peak negative LAε (LAε negative ), which corresponded to LA contractile function; peak positive LAε (LAε positive ), which corresponded to LA conduit function; and the sum of these values, total LAε (LAε total ), which corresponded to LA reservoir function. All LAε measurements were made by individual research personnel (HM) blinded from data analyses. The inter- and intraobserver variabilities for LAε total were studied in a group of 15 randomly selected subjects by 1 observer repeated twice (HM) and independently by a second observer (AGB) who were unaware of the other’s measurements and of the study time point. The bias (mean difference) and limits of agreement (1.96 SD of difference) between the first and second measurements were determined.
Continuous variables are summarized as mean ± SD if normally distributed and as median and interquartile range if non-normally distributed. Normality was assessed by the Shapiro-Wilk W test. Spearman’s rank correlation method was used as a nonparametric measure of association between LAε and clinical and echocardiographic indexes. The Wilcoxon or Kruskal-Wallis rank-sums tests were used to compare differences in LAε across clinical categories, whereas proportions were compared using contingency table analysis. Independent predictors of LV diastolic dysfunction grade III were determined by multiple logistic regression analysis with candidate variables added to a model containing LV diastolic dysfunction stage III as the dependent variable and LAε, E to early diastolic velocity of the septal mitral annulus ratio (E/e′ septal), and LAVi as covariates. The Cox proportional hazards regression model was used to assess the clinical risk associated with increasing continuous standardized increments of LAε. The proportional hazards assumption was verified with log (time) versus log (−log [survival]) plots. Statistical analyses were performed using JMP 9.0.0 (SAS Institute, Cary, North Carolina). All p values reported are from 2-sided tests and a p value <0.05 was considered statistically significant.
Results
Of the 118 patients who had LA images of both 4- and 2-chamber views in ADEPT, 108 patients (91.5%) had LAε that could be measured in both apical views. The average frame rate of the clips for LAε analysis was 30 ± 0.9 frames/s. The coefficient of variation of intraobserver variability for LAε total was 10 ± 7%. The coefficient of variation of interobserver variability was 11 ± 6%. The bias and limits of agreement of intra- and interobserver variabilities were 0.4 ± 6.9% and 1.8 ± 7.0%, respectively. A comparison of the clinical characteristics of the 108 patients included in the study with those of the 10 patients with inadequate images for analysis is listed in Table 1 . Excluded patients had a similar risk profile. In our study cohort, LAε negative , ε positive , and ε total were all correlated with individual indexes of LV diastolic dysfunction ( Table 2 ). LAε negative and LAε positive correlated in an opposite direction with the LV diastolic function parameters. All LAε indexes increased across diastolic stage (p <0.001 for all). All LAε indexes correlated with plasma N-terminal pro-B-type natriuretic peptide levels. Receiver operator characteristic curve analysis was performed to assess the accuracy of LAε and LAVi in diagnosing LV diastolic dysfunction grade III ( Figure 2 ). LAε negative showed the highest accuracy for detecting grade III LV diastolic dysfunction followed by LAε total and LAε positive . For LAVi, area under the curve = 0.815, p <0.001. In multivariate logistic regression analysis, indexes of LAε negative and LAε total were associated with the presence of LV diastolic dysfunction grade III even after adjustment for E/e′ septal and LAVi ( Table 3 ).
| Variable | Study Patients (n = 108) | Excluded on the Basis of Imaging (n = 10) | p |
|---|---|---|---|
| Mean age (yrs) | 57 ± 15 | 59 ± 13 | 0.703 |
| Men | 83 (77%) | 7 (70%) | 0.634 |
| NYHA class III or IV | 45 (42%) | 4 (40%) | 0.900 |
| Ischemic origin | 48 (45%) | 4 (40%) | 0.767 |
| Heart rate (beats/min) | 75 ± 13 | 73 ± 11 | 0.887 |
| Systolic blood pressure (mm Hg) | 114 ± 20 | 107 ± 23 | 0.366 |
| Hypertension | 54 (51%) | 4 (40%) | 0.507 |
| Diabetes mellitus | 29 (27%) | 3 (30%) | 0.846 |
| Medications | |||
| ACE-I and/or ARBs | 96 (91%) | 10 (100%) | 0.169 |
| β Blockers | 62 (58%) | 4 (40%) | 0.276 |
| Spironolactone | 16 (16%) | 3 (30%) | 0.290 |
| Loop diuretics | 79 (75%) | 8 (80%) | 0.696 |
| Digoxin | 59 (59%) | 6 (60%) | 0.951 |
| Echocardiographic data | |||
| LA strain negative | −6.8 ± 4.4% | ||
| LA strain positive | 7.7 ± 5.7% | ||
| LA strain total | 14.5 ± 8.2% | ||
| LV ejection fraction (%) | 25 ± 6 | 28 ± 4 | 0.135 |
| LV end-diastolic dimension (cm) | 6.3 ± 0.9 | 6.1 ± 0.9 | 0.700 |
| LV end-diastolic volume index (ml/m 2 ) | 117 ± 40 | 101 ± 27 | 0.346 |
| LV end-systolic volume index (ml/m 2 ) | 88 ± 34 | 75 ± 22 | 0.373 |
| LV mass index (g/m 2 ) | 158 ± 47 | 139 ± 43 | 0.215 |
| LA volume index (ml/m 2 ) | 42 ± 15 | 30 ± 16 | 0.083 |
| Mitral flow early to late diastolic velocity ratio | 1.7 ± 1.4 | 1.6 ± 1.9 | 0.264 |
| Mitral deceleration time (ms) | 172 ± 51 | 197 ± 74 | 0.295 |
| Systolic velocity of the mitral annulus (cm/s) | 5.7 ± 2.0 | 5.1 ± 1.1 | 0.119 |
| Early diastolic velocity of the mitral annulus (cm/s) | 7.2 ± 4.5 | 4.7 ± 1.2 | 0.499 |
| Late diastolic velocity of the mitral annulus (cm/s) | 7.5 ± 3.2 | 7.2 ± 2.5 | 0.703 |
| Mitral flow early diastolic velocity to early diastolic velocity of the mitral annulus ratio | 20 ± 12 | 15 ± 8 | 0.191 |
| Laboratory data | |||
| N-terminal pro-B-type natriuretic peptide (pg/ml) | 1,095 [456, 3,436] | 1,268 [501, 2,088] | 0.645 |
| Variable | LA Strain negative | LA Strain positive | LA Strain total | |||
|---|---|---|---|---|---|---|
| r | p | r | p | r | p | |
| Age (yrs) | −0.01 | 0.896 | −0.02 | 0.842 | 0.01 | 0.969 |
| N-terminal pro-B-type natriuretic peptide (pg/ml) | 0.44 | <0.0001 | −0.40 | <0.0001 | −0.52 | <0.0001 |
| LV ejection fraction (%) | −0.35 | <0.001 | 0.51 | <0.0001 | 0.52 | <0.0001 |
| Mitral flow early to late diastolic velocity ratio | 0.71 | <0.0001 | −0.22 | 0.023 | −0.55 | <0.0001 |
| Mitral deceleration time (ms) | −0.68 | <0.0001 | 0.41 | <0.0001 | 0.64 | <0.0001 |
| Systolic velocity of the mitral annulus (cm/s) | −0.47 | <0.0001 | 0.48 | <0.0001 | 0.53 | <0.0001 |
| Early diastolic velocity of the mitral annulus (cm/s) | −0.17 | 0.084 | 0.19 | 0.055 | 0.18 | 0.065 |
| Late diastolic velocity of the mitral annulus (cm/s) | −0.71 | <0.0001 | 0.44 | <0.0001 | 0.68 | <0.0001 |
| Mitral flow early diastolic velocity to early diastolic velocity of the mitral annulus ratio | 0.53 | <0.0001 | −0.21 | 0.029 | −0.44 | <0.0001 |
| Pulmonary vein systolic to diastolic flow velocity ratio | −0.72 | <0.0001 | 0.31 | <0.001 | 0.60 | <0.0001 |
| LA volume index (ml/m 2 ) | 0.52 | <0.0001 | −0.36 | <0.001 | −0.54 | <0.0001 |




