High residual platelet reactivity (RPR) on clopidogrel treatment has been associated with increased risk for ischemic events during follow-up in patients with acute coronary syndromes. The aim of this study was to assess the incidence, predictors, and clinical consequences of high RPR in a large population of patients with non–ST-segment elevation acute coronary syndromes who underwent percutaneous coronary intervention and stenting. Overall, 833 patients received point-of-care testing of platelet inhibition 30 days after percutaneous coronary intervention. High RPR was diagnosed on the basis of P2Y 12 reaction units >230. The incidence and predictors of death, myocardial infarction, stroke, and serious bleeding events were assessed up to 1 year from the day of testing. P2Y 12 reaction units were normally distributed, and 264 patients were classified as poor responders (31.7%). Independent predictors of response to clopidogrel were male gender (odds ratio [OR] 1.51), age (OR 0.96), diabetes mellitus (OR 0.51), and use of proton pump inhibitors (OR 0.59). At 1 year, poor responders showed higher rates of death (4.6% vs 1.9%, p = 0.032) and serious bleeding events (4.9% vs 1.8%, p = 0.009) compared with good responders. After adjustment for confounders, high RPR did not emerge as an independent predictor of mortality (OR 0.57, 95% confidence interval [CI] 0.23 to 1.42, p = 0.23) or serious bleeding events (OR 0.61, 95% CI 0.25 to 1.52, p = 0.29). The results did not change using the a cut-off value for P2Y 12 reaction units of 208. In conclusion, 1/3 of patients with acute coronary syndromes who underwent percutaneous coronary intervention and stenting showed high on-treatment RPR on bedside monitoring. They had a worse prognosis, but the level of platelet inhibition was not independently associated with the incidence of ischemic or bleeding events.
Dual-antiplatelet therapy is a mainstay of medical treatment after acute coronary syndromes (ACS) and after percutaneous coronary intervention (PCI) with stent implantation. Thienopyridines such as clopidogrel are irreversible antagonists of the P2Y 12 receptor, 1 of the 2 adenosine diphosphate receptors expressed on the platelet’s membrane, which promotes platelets’ activation and aggregation. Over the years, evidence has accrued regarding a variable response to clopidogrel due to a combination of genetic, metabolic, and clinical factors. Clopidogrel resistance may occur in 20% to 30% of subjects, and a number of studies have consistently demonstrated that high residual platelet reactivity (RPR) during dual-antiplatelet treatment is associated with an increased risk for adverse ischemic events. Point-of-care assays of platelet function linked to clopidogrel resistance represent a reliable and easy way to measure RPR. Nevertheless, a number of issues remain unresolved, including the timing and selection of patients for testing, the identification of clinically meaningful cutoff values, and the management of patients with high RPR. An ideal therapeutic window of platelet inhibition has been hypothesized, defined as the best possible balance between ischemic risk reduction and bleeding hazard increase. The identification of such a “sweet spot” could be useful for tailoring the use of newer antiplatelet agents as well. The present study was designed to identify the predictors of high on-treatment RPR 30 days after the procedure and to investigate the relation between RPR and clinical and hemorrhagic events in a large unselected population of patients with non–ST-segment elevation ACS who underwent PCI.
Methods
This study was a multicenter, prospective registry. Six third-level, high-volume catheterization laboratories participated in the study from January 2008 to October 2011. All patients with non–ST-segment elevation who underwent PCI and stenting at participating centers were potentially eligible for enrolment. Patients were either pretreated with clopidogrel at the time of PCI or had received a bolus dose of clopidogrel 300 mg >6 hours earlier or 600 mg >2 hours before the procedure and then 75 mg/day for 12 months. Periprocedural pharmacologic treatment was standard of care. All patients who consented to participate received point-of-care testing of platelet inhibition using the VerifyNow (Accumetrics, Inc., San Diego, California) 30 days after the procedure, which is a time frame that allows a stabilization of on-treatment platelet reactivity. Patients and investigators were kept blind to the results of the test, and no modifications to the treatment were performed on the basis of these findings. Inclusion criteria were (1) residence in the Emilia-Romagna region, (2) non–ST-segment elevation ACS treated by PCI and stenting in a de novo lesion, and (3) release of written informed consent. Exclusion criteria were (1) stable coronary artery disease, (2) use of oral anticoagulant therapy, (3) treatment with ticlopidine, ticagrelor, or prasugrel, (4) in-stent restenosis, (5) transfer from a peripheral hospital (no outpatient clinic visit scheduled at participating institutions), and (6) denial of consent.
Point-of-care testing of RPR was performed using the VerifyNow P2Y 12 , which is designed to measure blocking of the platelet receptor P2Y 12 after adenosine diphosphate stimulus. The instrument provides results in terms of P2Y 12 reaction units (PRU) and percentage of platelet inhibition.
Demographics and baseline clinical and procedural characteristics were collected in the Registro Angioplastiche dell’Emilia Romagna (REAL) database, which was previously described. On the basis of available data, the prespecified cutoff value of PRU between responders and poor responders to clopidogrel was 230 (poor responders >230 PRU). The occurrence of the following events was recorded starting from the date of the VerifyNow test: death, myocardial infarction, stroke, new revascularization (PCI and coronary artery bypass grafting), definite or probable stent thrombosis (per the Academic Research Consortium definition), serious bleeding events (SBEs). Major adverse cardiovascular events were defined as the combination of death, myocardial infarction, and stroke. SBEs were defined as intracranial or intraocular hemorrhage, any bleeding requiring hospitalization or red blood cell transfusion, or the need for intervention.
Follow-up information was obtained directly and independently from the Emilia-Romagna Regional Health Care Agency through the analysis of hospital discharge records and mortality registries. This procedure ensured complete follow-up for 96.9% of the patients resident in the region, including all out-of-hospital deaths (patients living outside the region were excluded upfront). Telephone interviews with patients or their relatives, clinical files, and outpatient clinic files were retrieved if deemed necessary to adjudicate clinical events.
Continuous variables were compared using Student’s t test and are expressed as mean ± SD. Categorical variables were compared using chi-square or Fisher’s exact tests as appropriate and are expressed as numbers and relative percentages. The cumulative incidence of adverse events was estimated using Kaplan-Meier method and compared using the log-rank test. Multivariate logistic regression analysis using all variables listed in Table 1 was done to identify independent predictors of effective inhibition of platelet aggregation by clopidogrel. The multivariate model was created using stepwise regression, in which 4 variables were always kept in the model (age, gender, use of proton pump inhibitors [PPIs], and diagnosis at admission), and other variables were entered into the model at the 0.20 significance level and removed at the 0.10 level. Model discrimination was measured using the C-statistic and the Hosmer-Lemeshow goodness-of-fit test. All tests were 2 sided, and statistical significance was defined as p <0.05. Stepwise multivariate analyses with the same method were used to identify predictors of mortality and bleeding events during follow-up. All analyses were performed with the SAS v9.2 (SAS Institute, Cary, North Carolina). The study was conducted according to the Declaration of Helsinki. The local medical ethics committee approved the protocol and written informed consent was obtained from every patient.
| Variable | All Patients | Responders | Poor Responders | p Value ∗ |
|---|---|---|---|---|
| (n = 833) | (n = 569) | (n = 264) | ||
| Age (yrs) | 67.6 ± 11.7 | 65.8 ± 11.8 | 71.6 ± 10.5 | <0.001 |
| Men | 630 (75.6%) | 453 (79.6%) | 177 (67.1%) | <0.001 |
| Body mass index (kg/m 2 ) | 27.4 ± 4.0 | 27.4 ± 3.9 | 27.4 ± 4.4 | 0.926 |
| Smokers | 178 (21.4%) | 138 (24.3%) | 40 (15.3%) | <0.001 |
| Diabetes mellitus | 239 (28.7%) | 136 (23.9%) | 103 (39.2%) | <0.001 |
| High cholesterol † | 562 (67.5%) | 400 (70.3%) | 162 (61.4%) | 0.010 |
| Hypertension | 577 (69.3%) | 388 (68.2%) | 189 (71.6%) | 0.322 |
| Chronic obstructive pulmonary disease | 91 (10.9%) | 51 (9.0%) | 40 (15.2%) | 0.008 |
| Previous myocardial infarction | 266 (31.9%) | 183 (32.2%) | 83 (31.4%) | 0.835 |
| Previous PCI | 212 (25.5%) | 149 (26.2%) | 63 (23.9%) | 0.474 |
| Previous coronary artery bypass | 55 (6.6%) | 40 (7.0%) | 15 (5.7%) | 0.466 |
| Anemia ‡ | 12 (1.4%) | 4 (0.7%) | 8 (3.0%) | 0.009 |
| Renal failure | 75 (9.0%) | 42 (7.4%) | 33 (12.5%) | 0.016 |
| Dialysis | 4 (0.5%) | 0 | 4 (1.5%) | 0.010 |
| Left ventricular ejection fraction <35% | 17 (2.0%) | 12 (2.1%) | 5 (1.9%) | 0.999 |
| Clinical presentation | 0.931 | |||
| Unstable angina pectoris | 371 (45.5%) | 254 (44.6%) | 117 (44.3%) | |
| Non–ST-segment elevation myocardial infarction | 462 (55.5%) | 315 (55.4%) | 147 (55.7%) | |
| Previous heart failure | 74 (8.9%) | 42 (7.4%) | 32 (12.1%) | 0.026 |
| Previous cancer | 58 (7.0%) | 39 (6.9%) | 19 (7.2%) | 0.856 |
| Multivessel coronary disease | 492 (59.1%) | 337 (59.2%) | 155 (58.7%) | 0.888 |
| Use of PPIs § | 326 (39.1%) | 200 (35.2%) | 126 (47.7%) | <0.001 |
∗ For comparison between subgroups.
† Total cholesterol >200 mg/dl, low-density lipoprotein >130 mg/dl, or use of cholesterol-lowering medications.
‡ Serum hemoglobin <13 g/dl for men and <12 g/dl for women.
Results
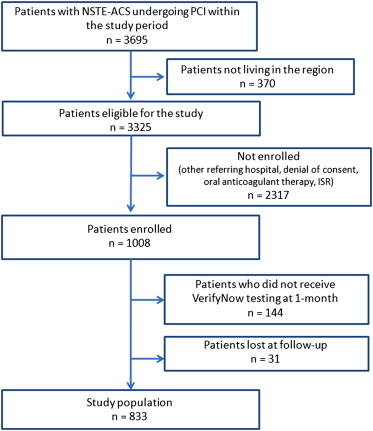
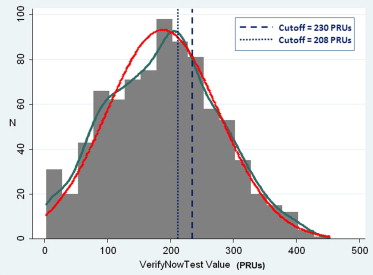
Study flow is shown in Figure 1 . During the study period, 3,695 patients with non–ST-segment elevation ACS underwent PCI at the 6 participating centers. After the exclusion of patients who did not meet enrollment criteria and those who did not comply with the study protocol, the final study population consisted of 833 subjects. The distribution of VerifyNow results expressed as PRU is shown in Figure 2 . As expected, PRU was normally distributed (Shapiro-Wilk test W = 0.993, p = 0.001). On the basis of the prespecified 230 PRU cutoff value, 569 patients (68.3%) were classified as responders and 264 as poor responders (31.7%). Table 1 lists the demographic and clinical and procedural characteristics of all patients enrolled and divided between responders and poor responders. Table 2 lists the angiographic and procedural characteristics. Compared with responders, poor responders were older; were less frequently male; had more co-morbidities such as diabetes, chronic obstructive pulmonary disease, anemia, renal failure, and heart failure; and were more often using PPIs. Conversely, there were no significant angiographic or procedural differences ( Table 2 ).
| Variable | All Lesions | Responders | Poor Responders | p Value ∗ |
|---|---|---|---|---|
| (n = 1,174 † ) | (n = 831) | (n = 343) | ||
| Coronary artery | ||||
| Left main | 4.2% | 3.1% | 4.7% | 0.1979 |
| Unprotected | 3.0% | 2.5% | 4.1% | 0.1546 |
| Left anterior descending | 40.2% | 41.3% | 37.6% | 0.2443 |
| Proximal | 14.9% | 15.3% | 14.0% | 0.5733 |
| Left circumflex | 30.3% | 30.2% | 30.6% | 0.8902 |
| Right | 25.9% | 25.4% | 27.1% | 0.5405 |
| Bypass graft | 2.2% | 2.0% | 2.6% | 0.5408 |
| Venous | 2.1% | 1.9% | 2.6% | 0.4513 |
| Arterial | 0.1% | 0.1% | 0 | 0.5208 |
| Lesion type (Ellis) | ||||
| A | 6.5% | 6.9% | 5.4% | 0.3822 |
| B1 | 28.0% | 28.4% | 26.9% | 0.6155 |
| B2 | 42.7% | 42.3% | 43.5% | 0.7185 |
| C | 9.2% | 8.6% | 10.5% | 0.3304 |
| Bifurcation | 23.6% | 22.4% | 26.3% | 0.1913 |
| Total occlusion | 6.1% | 5.8% | 6.8% | 0.5120 |
| Ostial lesion | 10.9% | 11.4% | 9.7% | 0.4115 |
| Lesion length (mm) | 17.1 ± 9.1 | 16.9 ± 9.0 | 17.5 ± 9.5 | 0.3417 |
| >20 | 32.8% | 33.0% | 32.0% | 0.7589 |
| >30 | 8.9% | 9.3% | 7.8% | 0.4649 |
| Reference vessel diameter (mm) | 2.9 ± 0.5 | 2.9 ± 0.5 | 3.0 ± 0.5 | 0.1363 |
| <2.50 | 35.1% | 36.5% | 31.6% | 0.1442 |
∗ For comparison between subgroups.
In the logistic regression analysis, independent predictors of effective response to clopidogrel were male gender, age, diabetes mellitus, and use of PPIs ( Table 3 ), with male gender associated with a greater likelihood of effective response (PRU ≤230) and age, diabetes, and the use of PPIs associated with increased likelihood of ineffective response.
| Covariate | OR | 95% CI | p Value |
|---|---|---|---|
| Male gender (reference: female) | 1.51 | 1.06–2.14 | 0.022 |
| Age (continuous variable) | 0.96 | 0.94–0.97 | <0.001 |
| Non–ST-segment elevation myocardial infarction (reference: unstable angina) | 0.92 | 0.67–1.26 | 0.597 |
| Use of PPIs | 0.59 | 0.43–0.81 | <0.001 |
| Diabetes mellitus | 0.51 | 0.36–0.70 | <0.001 |
| Previous bypass | 1.74 | 0.90–3.37 | 0.098 |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


